QM software for your document & quality management » Digital LS
Modular. Holistic. Effective.
Your path to digitalisation
The digital transformation opens up new opportunities for companies to increase their efficiency and transparency. With our eQMS suite, we offer you holistic solutions for document management, employee training and other process optimisations. The eQMS suite not only fulfills the regulatory requirements, but also supports you in preparing for audits. Tailored to your employees and processes, our QM software enables effective management of documents and quality measures.
Our modular system
The digitalisation solutions are specifically designed to efficiently optimise document-based processes in quality management and manufacturing/production. The eQMS suite (dls | eQMS) is based on an integrated ECM/DMS system. By adding non-GxP applications and connecting to your existing ERP system, e.g. SAP, almost all document-based business processes can be mapped.
Our software solutions can be introduced on a modular basis and expanded like a building block model to form a complete GxP-compliant eQMS suite. This is illustrated by the building blocks highlighted in green.

eQMS-Suite - your key to digital transformation in quality management
Get a comprehensive insight into the world of digital transformation with our eQMS suite. Find out more about Digital Life Sciences and our solutions in this video. Discover how you can redesign your document-based processes with customised, GxP-compliant software solutions and immerse yourself in the many possibilities of digital transformation.
You are currently viewing a placeholder content from YouTube. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More Information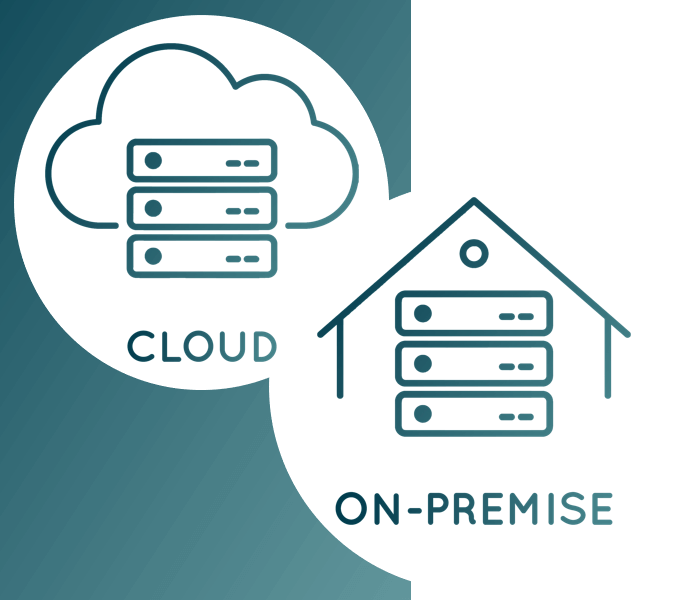
eDMS | eQMS - Cloud or on-premise? Both!
In order to be able to offer you the ideal mode of operation for our software solutions, we are strategically aiming to give you, our customers, the choice.
It is very important to us that you can decide for yourself which deployment method best suits your needs. In addition to classic on-premise operation, in which the eDMS | eQMS Suite is operated on your own infrastructure, we also offer you the alternative of using our suite in the cloud. This is available to you either as Software as a Service (SaaS) or as an Application Service Provider (ASP) in a state-of-the-art and secure data centre.
Our data centre partner is accessible to customers and interested parties and has many years of GxP know-how. The storage and mirroring of the data naturally takes place in Germany, more precisely in Hamburg. The qualified data centre meets all current IT security standards and is distinguished by certifications and conformities such as ISO 27001, FDA 21 Part 11 Compliance, GxP Compliance and others.
Get a compact short overview

Document Control software
Whether work / process instructions (SOPs), process descriptions, test specifications or other document types — you create, revise and sign them all digitally with the Document Control software.

Training Management software
Extend the module “Document Control” to actively plan and log your employees' qualifications with our training management software.

E-Learning software
Use the new “E-learning” software to train your employees digitally. Create an e-learning course according to your wishes using Microsoft PowerPoint or integrate existing presentations.

Document Control software
Whether work / process instructions (SOPs), process descriptions, test specifications or other document types — you create, revise and sign them all digitally with the Document Control software.

Training Management software
Extend the module “Document Control” to actively plan and log your employees' qualifications with our training management software.

E-learning software
Use the new “E-learning” software to train your employees digitally. Create an e-learning course according to your wishes using Microsoft PowerPoint or integrate existing presentations.
QM processes (Complaint | DC | CAPA | CC)
Digitalise your ISO processes. Control your production-related QM processes using digital workflows.

QM processes (Complaint | DC | CAPA | CC)
Digitalise your ISO processes. Control your production-related QM processes using digital workflows.

Technical Documentation software
Create and update your technical documentation, e.g. in accordance with the requirements of the Medical Device Regulation (MDR).

Contract Management software
Keep track of your contracts. Get reminders for deadlines like termination or renewal options in a multi-stage process.

Technical Documentation software
Create and update your technical documentation, e.g. in accordance with the requirements of the Medical Device Regulation (MDR).

Contract Management software
Keep track of your contracts. Receive reminders for deadlines like termination or renewal options in a multi-stage process.
Frequently asked questions (FAQs) about the quality management system
What are the specific requirements that GxP places on quality management?
As an answer, the German GMP regulations, AMWHV, help us: The quality management system (QM system) is a system that includes quality assurance, good manufacturing practice or good work practice, including quality control and periodic product quality review (PQR) (§ 2 Definitions).
§3 Quality Management System, Good Manufacturing Practice and Good Professional Practice
Companies and facilities must implement a company quality management system (QM system) that corresponds to the type and scope of the activities performed. The QM system shall include good manufacturing practice in the cases referred to in paragraph 2 and good professional practice in the cases referred to in paragraph 3, and shall provide for the active participation of the management of the establishments and facilities and of the personnel of the various sectors concerned. All areas involved in establishing, maintaining and implementing the quality management system must have competent personnel and suitable and sufficient premises and equipment. The quality management system must be fully documented and its proper functioning demonstrated (§ 1).
For which industries does the introduction of a quality management system make sense?
Certified companies have long been the norm in manufacturing and industries with many production steps. However, it is also a fact that it’s increasingly used in the service sector. Managers have understood that process and “product” quality are also methods in this area to differentiate their own product from that of the competition. Furthermore, process quality is an effective way to avoid the cost of errors. That is why more and more companies in the service sector are relying on the DIN EN ISO 9001 quality management standard. The more specific the industry, the more specific the standard. For example, laboratories have an ISO 17025 system, the automotive industry has the IATF 16949 standard, and medical device manufacturers have the ISO 13485 standard. These exemplary standards are all derived from ISO 9001. However, due to their respective fields, they often have more extensive requirements and more specific bases.
What are the benefits of a quality management system?
A quality management system (QMS) has many advantages. It can lead to cost savings and facilitate market access. A quality management system helps companies in the following essential aspects:
- Improved market access: In a globalised market environment, a company with a certified QMS can demonstrate that quality and process safety are systematically addressed. In some industries, such as the automotive industry, such a certificate is a mandatory requirement for business transactions.
- Better quality at lower cost: Quality management ensures quality as early as possible in the development process. Early detection of defects is much more cost effective compared to detection during final inspection.
- Cost savings: The company saves money by producing less waste. This reduces the cost of purchasing raw materials and disposing of waste.
- Systematic quality thinking among employees: A QMS allows all employees of a company (not only the quality assurance experts) to be involved in quality assurance.
- Transparent business processes: Operational processes are documented and described. This makes them transparent and easier to review for improvement opportunities. It also makes it easier to identify and eliminate problems.
- Sustainable standard: Quality management systems help to secure the achieved optimisations in the long term by making the improved procedures and processes “standard practice” and thus the norm.
- Better transfer of know-how: Documented and described workflows and processes enable a more efficient and effective transfer of operational know-how. This makes it easier to train new employees, for example.
What is QM software?
Quality management software (QM software) is a digital system that supports companies in optimising and automating their quality management processes. These software solutions offer a variety of functions that make it possible to define and monitor quality standards and ensure that they are adhered to. The main features include document management, deviation control, change control and the management of corrective and preventive actions (CAPA).
QM software supports companies in meeting regulatory requirements such as ISO 15189, 17025, 9001, 14001, 16949, FDA 21 CFR Part 11, MPG, ISO 13485, MDR 2017/745 and many more. It enables centralised storage and management of documents such as standard operating procedures (SOPs), quality management manuals (QMH) and other relevant evidence. By automating processes, the software increases efficiency and transparency, reduces sources of error and improves traceability.
QM software also promotes digital transformation in companies by making it possible to convert manual and paper-based processes into digital workflows. This leads to significant time savings and improved collaboration between different departments. Overall, QM software helps to sustainably raise quality standards in the life sciences sector.
What is a QM system?
A quality management system (QM system) is a structured framework of processes, procedures and responsibilities aimed at continuously ensuring and improving the quality of products and services. It is based on the principles of quality assurance and quality control, which ensure that the specified standards and requirements are met.
An effective QM system comprises various elements, including the documentation of work processes, the implementation of standard operating procedures (SOPs), the performance of internal audits and the definition of corrective and preventive actions (CAPA) to handle deviations. It helps companies to comply with regulatory requirements such as FDA 21 CFR Part 11 or the EU GMP guidelines and promotes a culture of continuous improvement.
By using a QM system, companies can increase efficiency and transparency in their processes, minimise risks and increase customer satisfaction. Digital transformation, supported by electronic quality management systems (eQMS), enables seamless management and analysis of quality data, leading to optimised decision-making. A QM system is therefore an indispensable tool for companies in the life science sector.
Where can our QM software support?
With the QM software from Digital Life Sciences, you receive a system at the highest level relying on the excellent process knowledge and expertise of the team. You get software that is open for the integration of other IT systems, such as LIMS/ERP. Due to the continuous further development as standard software, you are perfectly prepared with regard to the validation of the software and furthermore remain flexible with regard to your customisation wishes due to individual configuration options. Moreover, our solutions can be understood as a modular building block system, where you can successively expand your system.
With the quality management software from Digital Life Sciences…
- you manage your specification documents (e.g. work and process instructions, test specifications, hygiene plans), forms, contracts and other document types digitally
- you take care of document distribution and signature circulation digitally at the touch of a button and focus on your core business
- you protect your data from unwanted actions of unauthorised users
- save time by minimizing process runtimes for creating, revising, releasing, and distributing your default documents
- you reduce unnecessary printouts and paper on your desk. This preserves the environment and your nerves as well as saves you money.
What are the benefits of quality management?
Quality management (QM) is a systematic approach to ensuring that products and services meet the defined quality standards. It promotes the continuous improvement of processes, products and services, thereby increasing efficiency and transparency within a company. By implementing a quality management system (QMS), companies can ensure that they comply with regulatory requirements such as FDA 21 CFR Part 11 and the EU GMP guidelines.
Effective QM minimises the risk of deviations and errors as it defines clear procedures and guidelines for quality assurance. It also enables structured deviation control and change control, which are crucial for maintaining product integrity. QM also supports the documentation and traceability of all relevant processes, which is essential for audits and inspections.
By focusing on quality and customer satisfaction, QM helps to strengthen confidence in the company and its products, which can ultimately lead to an improvement of the market position. The digital transformation in QM, for example through the use of an electronic quality management system (eQMS), further optimises these processes and promotes efficiency throughout the entire organisation.
Does our QM software meet the requirements of an audit?
Our products were launched and validated successfully in numerous pharmaceutical companies. Likewise, our products have been successfully audited during TÜV audits in accordance with the guidelines of ISO standards 9001 and 13485 and during inspections by the Regional Council (Germany).
As a software provider for companies in the GxP environment, conducting supplier audits is common practice. In recent years, we have already been audited many times in on-site audits or postal audits by our customers and interested parties, e.g. in accordance with the requirements of GAMP5.
What advantages does digitalisation offer in quality management?
Digitalisation in quality management offers numerous advantages and has established itself as an indispensable part of modern business processes. The potential of digitalisation is particularly evident in the area of document control and the use of quality management software (QM software). Some key advantages are highlighted below.
- Efficient document control: One of the most important aspects of digitalisation in quality management is optimised document control. By using QM document management software, documents are managed centrally and can be accessed at any time. This enables a simpler and faster search as well as better traceability of changes. Documents can be updated and new versions released directly in the software, ensuring compliance with regulations.
- Quality improvement through QM software: Quality management software (QM software) offers specialised functions that enable comprehensive monitoring and control of quality processes. This includes the management of quality assurance measures. With the help of a well-structured quality management program, companies can make a significant contribution to increasing efficiency and improving quality. QM training courses, often supported by presentations such as a QM training PowerPoint, are also simplified and standardised by such software products.
- Easy handling and integration: Another advantage of digitalisation in quality management is the simple integration of software solutions into existing systems. The quality management software can be easily integrated into different IT structures, which reduces the effort required for implementation and employee training.
- Standardisation and transparency: The use of QM system software and quality management software not only guarantees standardised workflows, but also ensures greater transparency across all processes. This is particularly important for the documentation and traceability of quality-relevant measures. Standardised processes and the central management of documents increase efficiency and minimise sources of error.
- Support with technical documentation: By using technical documentation software, complex technical documents can be created and managed easily and efficiently. This is particularly useful in industries with high regulatory requirements. The documentation of technical specifications and processes is therefore not only precise, but also audit-compliant and traceable.
- Digitalisation as the key to the future:
Digitalisation in quality management offers decisive advantages. From the simple handling and management of documents to the integration of specialised software solutions and the fulfillment of complex documentation and traceability requirements – the possibilities are manifold. By implementing QM software, the efficient use of document control software and the use of specialised technical documentation software, companies can achieve significant improvements in their quality processes. The digital future of quality management will be characterised by transparency, efficiency and compliance, and the ongoing digitalisation of quality management provides the ideal basis for this. Companies that take digitalisation in quality management seriously are laying a solid foundation for efficient and legally compliant work in an increasingly digital world.
Get consultancy now
You have a question about our solutions or would like to know more?
Let us analyse and transform your current processes together.
Attention: Form is
currently not supported by:
⛔ iPhones with iOS version <14