CHANGE MANAGEMENT SOFTWARE
Digitize your change management
Optimize the efficiency of your change processes by using automated, digital workflows. Guarantee the quality of your products and services and ensure that all internal and legal requirements are reliably met.
Automated forwarding and escalation management
Consideration of internal and legal requirements
Real-time overview of all deviation processes

You are currently viewing a placeholder content from YouTube. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More Information» dls | change control «
Software for your change processes
Optimize your QM processes in the pane of change control (Change Control, CC) with our advanced digital workflows. Our integrated solution completely transforms your previously paper-based process into a digital format, from recording to completion.
The workflow system ensures that the digital change form is automatically forwarded to the next instance, which increases efficiency and traceability. If time is exceeded, our comprehensive reminder and escalation management system is activated so that you always have an overview. In addition, a regulation for substitutions is integrated into the task routing to seamlessly compensate for failures and ensure the continuity of your processes.
What benefits does the Change Control software offer you?
Regulatory Compliance:
Changes to products, processes or systems are made in accordance with relevant regulatory and internal requirements.
Continuous data availability
Access your data at any time and from anywhere to ensure maximum flexibility.
Increased data security
Protect your data and information from unauthorized access and external threats.
Individual configuration
Customise the modules to your specific requirements to make it easier for your employees to complete the onboarding training.
Process safety
The workflow system ensures that each step of the CC process is forwarded to the appropriate contact person. The escalation system takes effect in the event of delays.
Cost reduction
Administrative costs and throughput times are significantly reduced, while the quality of the results increases at the same time.
Integration into existing processes
Change Controls can be initiated, for example, from deviation reports (Deviation Control) or corrective and preventive actions (CAPAs).
Transparency
Every authorized employee has insight into the current status of all CC processes, regardless of their direct involvement.
Central information platform
Use d.velop documents (formerly d.3ecm) as a central platform for quick access to all relevant data.
Reports
Create comprehensive reports and statistics on all change control processes.
Get to know our software better
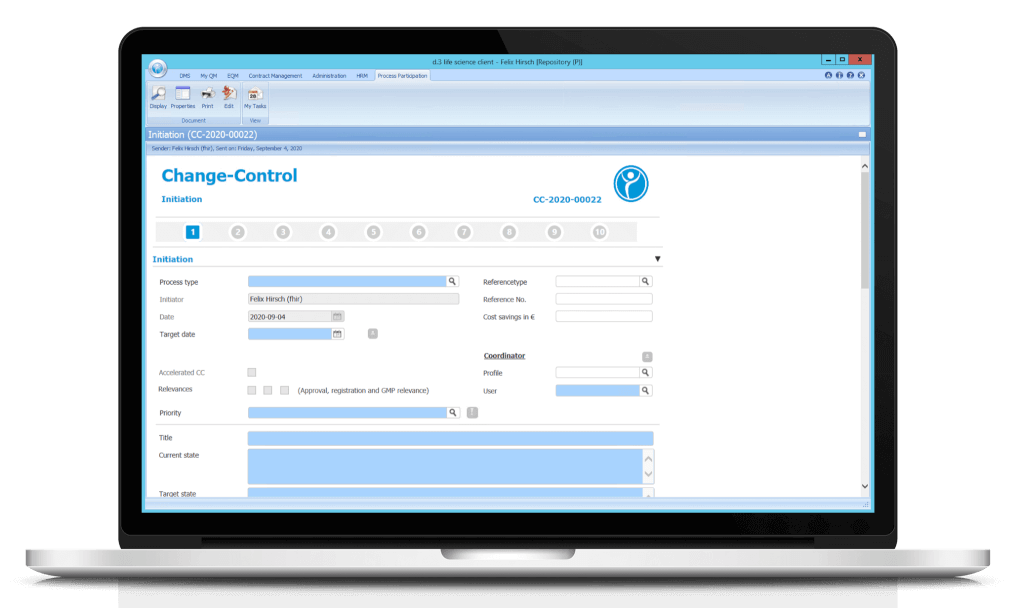
The digital forms are also converted to a PDF/A in parallel. This way, employees who are not involved in the process can also gain insight into the workflow and the document can be shared with external parties if required.
What is a Change Control process?
The Change Control process is used to ensure that changes affecting products, processes and systems are carried out in conformity with regulatory and internal specifications. The aim of this process is to provide a systematic, traceable changes management.
Every action in the process is automatically documented by the system accordingly. Integrated audit trail functions ensure high traceability.
The digital form is filled out step by step and provided with the relevant entries and data. Fields with a blue background are mandatory. Predefined value sets and drop-down functions make it easy for users to fill out the form.
After processing the individual steps (1. Initiation & categorization, 2. Evaluation of relevance for approval, 3. Evaluation of relevance for registration, etc.), the workflow system automatically sends tasks and messages to the responsible agents or workflow participants. Once all steps have been processed, the workflow is considered complete.
1. Step: Initiation
The initiation of the Change Control is done by the process responsible of the concerned department. He or she records the relevant data for Change Control. The process is then forwarded to the appointed Change Control responsible.
Would you like a live insight into the software?
Get a comprehensive live insight into the possibilities of production-related quality management processes within just 45 minutes using a specific use case. Learn how you can use Digital Life Sciences solutions to optimize your production-related QM processes, such as
complaints (Complaint Management), deviation reports (Deviation Control), corrective and preventive actions (CAPA) and change control (Change Control) efficiently through our digital workflows.
Some features of Change Management
- Classification of the Change Control processes according to types
- Evaluation of a CC according to approval, registration or GMP relevance with consequent dealing in the dynamic CC form
- Distributor for planning, processing and approving measures
- Optional return in process
- Logging of activities in audit trail
- Parallel and serial sending of tasks
- Automatic PDF creation and storage of the form in the eDMS after each step
- Referencing master data from your ERP system
- Add documents such as digital photos to your CC form
- Initiation of the individual tasks for the further processing of the workflow
- Escalation messages both within the system and by e‑mail
- Integrated absence management
- Initiation of a document change request for a controlled document
Regulations fulfilled by the Change Control process
33 Good reasons for a cooperation with Digital LS
You’re not convinced yet? You’re not convinced yet? Find out about 33 good reasons speaking for a cooperation with Digital Life Sciences GmbH. We will show you reasons from the provider’s point of view, from the software point of view and other general reasons that distinguish us.
Customer review on the Change Management
“Digital Life Sciences GmbH has supported us in the changeover to a new electronic quality management system in record time with professionalism and expertise. Six independent document management systems were transferred into one harmonized central d.3 eQMS. The support in implementing the validation requirements for computer-based systems according to GxP and ISO requirements is excellent. The modules “Document Control”, “Employee Qualification”, “Deviation Control”, “CAPA” and “Change Control” are globally operated at SCHOTT AG and by integrating additional tools in quality management, the portfolio could be improved beyond Document Control. We are building on the positive experiences of the cooperation and will continue the good partnership and good cooperation with other topics."

You might also like…
Change management is a component of the Digital Life Sciences solution suite. Each product is powerful on its own, but when used together they are even better.

Document Control software
Whether work / process instructions (SOPs), process descriptions, test specifications or other types of documents — you can create, revise and sign them all digitally with the document control software.

Training Management software
Extend the “Document Control” module to actively plan and record the qualifications of your employees with our training management software.

E‑learning software
Use the new software “E‑Learning” to train your employees digitally. Create an e‑learning course according to your wishes using Microsoft PowerPoint or integrate existing presentations.
Frequently asked questions (FAQs) about Change Control
Can attachments be stored with a Change Control?
If additional files are required for a process, attachments can be uploaded in all process steps and linked to the current process. The attachments are stored under a corresponding document type in the DMS and can be viewed in the Change Control under ‘Attachments’ by all process participants. You can configure which file types can be saved as attachments and how large the files can be.
Can the predefined designations be changed?
In the workflow administration, you can configure the names of the process steps, field names and datasets.
Can process steps be subsequently corrected?
In principle, a process step is completed with an electronic signature and can no longer be corrected. With the function ‘Step back’ the process can be returned to a previous process step. The user who has completed this step receives it again for editing and the process continues from this step. Any return to a previous step is recorded in the audit trail.
Can I analyze the Change Controls?
The essential data of a Change Control is transferred to the attributes of the data record. This allows you to search and analyze the Change Controls you have created. The analyses can be exported as PDF, EXCEL or WORD documents.
Are there templates for measures in a Change Control?
Having entered a measure, you can save it as a template in the measure table. The templates are available for insertion in a Change Control. The due date for the measure is calculated based on the duration entered in the template.
What is Change Control / Change Management?
Change Management / Change Control (CC) is a formal process within a quality management system. The aim is to ensure that all changes to a product or process are carried out in a controlled and coordinated manner and that the quality of the product is maintained despite the change.
Is there a use case example for the Change Control?
The equipment in the GMP cleanrooms must be qualified and the processes used must be validated. This means that it is defined and documented in advance which equipment is used in the manufacture of a product, how the process parameters are to be set, which ingredients are required, and much more. For example, changes can be made to devices or also processes:
- The system parameters must be changed.
- Parts or components of the plant must be renewed.
- Ingredients or quantities changed.
- Cleaning or testing procedures changed.
What is the definition of change control in quality management and why is it important?
Change control in quality management is a systematic process that ensures that all changes are implemented and documented within a controlled and traceable framework. This process is key to ensuring consistency, transparency and product quality as well as compliance with regulatory requirements.
How does change control software support the change control process in a corporate environment?
Change control software optimizes the change control process by automating and seamlessly documenting all the necessary steps. This software enables companies to plan, implement and monitor changes efficiently, improving traceability and facilitating compliance. This is particularly important in regulated sectors such as the pharmaceutical industry, where precise processes are essential.
What role does a change control manager play in a change management system?
A change control manager is responsible for monitoring and coordinating the change management system. His main tasks include systematically evaluating changes, working closely with various departments and ensuring that all relevant procedures and processes are complied with in accordance with regulatory requirements. The manager has a key responsibility to ensure that changes do not compromise the integrity and quality of the product or service, ensuring continued compliance with regulations.
Are there time limits for the execution of the process steps?
In the administration, time specifications can be entered for each process step. Reminder messages are sent before the process steps are due. The reminder message is sent to the processor of the task, the coordinator and the configured group that is to be informed about the reminder (for example, QA). A reminder is sent with the configured subject.
Can a process be cancelled?
It can be configured so that the process can be cancelled at any process step. For the cancellation, it is required to indicate a reason in a mandatory field. Only the coordinator of the process is allowed to cancel.
Can tasks be delegated?
In all process steps that follow the initiation, you can forward the process step to another person. The recipient’s authorization is checked during the selection.
Are the contents of all process steps visible?
When working on a process step, only this step can be processed, but the other process steps are still visible.
Can the predefined steps be customized?
The Change Control process flow has been designed in accordance with the GxP regulations and created in consultation with QM experts. Against the background of the validation documentation, the process steps are therefore mandatory. However, each process step contains a ‘Custom Panel’, which can be used to display further information or initiate actions.
What is the use for Change Control / Change Management?
In the pharmaceutical industry, but also in medicine, the qualification is a prerequisite for a manufacturing license. Significant changes to qualified equipment always result in subsequent re-qualification. Those responsible must decide whether changes are necessary based on a risk analysis. In addition, the question must be answered as to how the change affects the quality of the product and which qualification tests must be repeated.
What are the arguments in favor of using a change management software?
Compliance with partial regulatory requirements is simplified or made possible in the first place, and the corresponding risks are minimized. Change processes are standardized and traceable. Documentation requirements can be met by default. Employees at different sites can work on the same change without having to wait for documents by mail.
What are the main components of a change control form and how is it used in the change control process?
A change control form is a crucial document in the context of change management and contains essential information, including a detailed description of the change, the underlying reasons, the sections affected and the necessary approvals. It serves as structured documentation in the change control process and ensures that all relevant approvals and checks are completed before the change is implemented in practice.
What are the main differences between change control and change management in production and how do they complement each other?
Although change control and change management are closely linked, they have different focuses that are of crucial importance in production. Change Control focuses on the systematic formalization and documentation of changes. The aim is to ensure that all changes are carefully evaluated and approved. This is done through specific procedures and forms aimed at ensuring consistency and compliance.
Change management is a comprehensive concept that encompasses the systematic planning, implementation and monitoring of changes in processes, technologies and organizational structures. In production, change management plays a decisive role in the introduction of new technologies and software systems. It ensures that all employees are adequately prepared and trained to ensure a smooth transition and optimize the efficiency of processes.
Change control and change management together form a comprehensive framework for change management in production. Change Control ensures structural integrity and compliance with regulatory requirements, while change management promotes the smooth and efficient implementation of changes.
Quick contact
Do you have a question about the Change Control module?
Our sales team will help you promptly and gladly.
