SUPPLIER QUALIFICATION SOFTWARE
Software for your effective supplier qualification
Qualifying your suppliers minimises the risk from outsourcing services and ensures the quality of your products, even in international supply chains.
Always up-to-date information about the status of your suppliers
Compliance with your specific regulations
Efficiency and transparency through digital processes

You are currently viewing a placeholder content from YouTube. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More Information» engamp® | supplier qualification «
Software for the effective qualification of your suppliers
Our software “engamp® | supplier qualification” effectively optimises the supplier qualification process by documenting risk-based procedures and providing a constantly updated overview of the status of your suppliers. This central source of information allows you to react quickly to changes and ensure compliance with quality standards, ultimately guaranteeing the quality of your end products.
By automating the qualification process, the supplier qualification software significantly reduces the administrative workload. It enables you to optimise the planning and implementation of audits, monitor the validity of certificates and ensure that all suppliers meet the necessary requirements. This increases your company’s efficiency by saving time and resources while minimising the risk of quality defects.
What benefits does the supplier qualification software offer?
‘Single point of truth’ regarding the status of your suppliers
Supplier qualification serves as an essential source of information for transparently and reliably determining the status of suppliers from whom services and materials may be procured.
Representation of the supply chain based on material numbers
The visual representation of the supply chain from materials to the end product creates greater transparency for stakeholders and enables a differentiated view by target country.
Risk-based definition of qualification measures
Defining the required documents based on your risk evaluation ensures compliance with regulatory requirements and avoids missing proofs.
Efficient planning and implementation of audits
Integrated tools for optimised planning and implementation of audits save time and resources and increase the overall efficiency of quality control processes.
Document dossier per qualification
A specific dossier with documents for each qualification documents the current status and enables direct access to proofs, certificates, contracts...
Monitoring of certificates and qualifications
Automatic monitoring and notification of the validity of certificates keep the certification status up to date, prevent interruptions in the supply chain due to expired certificates and help maintaining high-quality standards.
Templates for questionnaires, audit reports and evaluations
Predefined templates for different evaluation processes standardise data collection and reporting, facilitate audit preparation and conducting and increase efficiency.
We would like to present you some features already
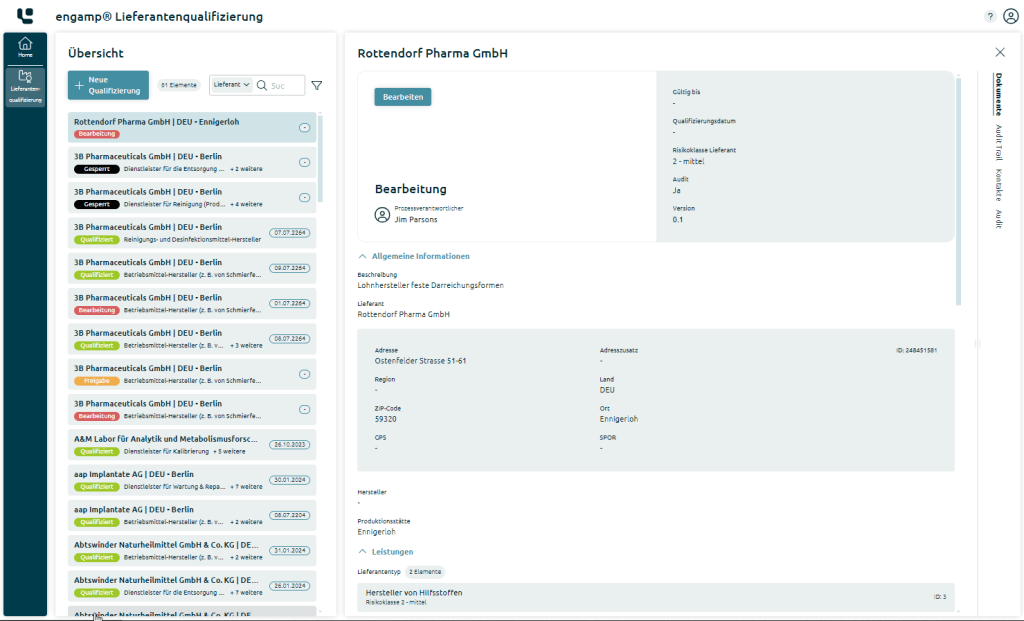
The list view of qualifications acts as a ‘single point of truth’ for the current status of your suppliers. Using quick filters for a risk class or status, you can search for specific suppliers, supplier types or material numbers.
The measures required to qualify a supplier type are determined and the validity period of the qualification is calculated taking into account the risk class.
The qualification documents are directly accessible as a dossier. The descriptive metadata continuously monitors the validity of the stored certificates and other proofs.
By assigning material numbers, products and target countries, you gain a comprehensive insight into a product’s supply chain.
Would you like a live insight into the software?
Get a live insight into the possibilities of qualifying suppliers in just 45 minutes based on a sample use case. Find out how you can use Digital Life Sciences solutions to obtain up-to-date information on the status of your suppliers.
Some features of supplier qualification
- ‘Single point of truth’ regarding the status of your suppliers
- Clear presentation of the supply chain based on material numbers in relation to the end products
- Specification of the required documents according to the risk classes of your suppliers
- Templates for questionnaires, audit reports, evaluations and much more
- Document dossier per qualification
- Efficient planning and management of audits
- Monitoring the validity of certificates and qualifications
The following regulations were taken into account in the supplier qualification software
GMP Guideline Chapter 1 & 5
MDR 2017/745 Article 10 (9) d
ISO 13485:2016 7.4.1
FDA 21 CFR 820.50
Ordinance for the Manufacture of Medicinal Products and Active Pharmaceutical Ingredients (AMWHV) § 11
ICH Q10 2.7
33 Good reasons for a cooperation with Digital LS
You’re not convinced yet? You’re not convinced yet? Find out about 33 good reasons speaking for a cooperation with Digital Life Sciences GmbH. We will show you reasons from the provider’s point of view, from the software point of view and other general reasons that distinguish us.
Customer opinion on supplier qualification
“Years ago, we used to track the status of documents such as work instructions, employee qualification records and batch reports using an Access database or on paper. However, it was tedious to keep the documents up to date and to manage them in an audit-compliant manner. In 2012, we switched to the digital document management of Digital Life Sciences, which was a quantum leap. Today we can create, find, edit and coordinate documents much faster. This saves working time and costs.“

You might also like…
Supplier qualification is a building block of the Digital Life Sciences solution suite. Each product is powerful on its own, but when used together they are even better.

Document Control software
Whether work / process instructions (SOPs), process descriptions, test specifications or other types of documents — you can create, revise and sign them all digitally with the document control software.

E‑learning software
Use the new software “E‑Learning” to train your employees digitally. Create an e‑learning course according to your wishes using Microsoft PowerPoint or integrate existing presentations.
QM process (Complaint | DC | CAPA | CC)
Digitalize your ISO processes. Control your production-related QM processes using digital workflows.
Frequently asked questions (FAQs) about supplier qualification
What does supplier qualification mean?
Supplier qualification is the process of evaluating and selecting suppliers to ensure that they meet the company’s quality and compliance requirements. This process involves several steps, including risk evaluation, conducting audits, reviewing regulatory compliance and regularly monitoring the supplier’s performance. The goal is to guarantee the integrity of the supply chain and ensure that materials and services meet the specified quality standards.
What does the supplier qualification process look like?
Supplier qualification is an essential part of quality management, especially in highly regulated sectors such as the life science industry. It ensures that all suppliers comply with the required standards and legal regulations. The supplier qualification process can be described as follows:
1. Needs analysis and recording of requirements:
- Definition of the type and quality of raw materials and packaging materials, taking into account the finished products.
- In the case of procurement via intermediaries, responsibilities must be defined and the source of supply identified.
2. Supplier selection:
- Research and compilation of potential suppliers.
- Pre-selection based on criteria such as product quality, price, delivery reliability and compliance with relevant regulations.
3. Risk evaluation:
- Risk classes are assigned to suppliers and service providers, possibly supplemented by a more detailed classification using a risk priority number.
The necessary GMP requirements are then defined.
4. Qualification:
Risk minimisation measures are defined for each identified risk:
- Request and evaluation of questionnaires and self-disclosure forms to clarify important information.
- Clarification of ambiguities with the supplier and documentation for the qualified person for the overall evaluation.
- Storage of certificates, references and testimonial from other pharmaceutical manufacturers.
- Material qualification based on manufacturer batches, which are checked for conformity with certifications and specifications.
- Quality agreement in which central aspects such as GMP standard, storage/transport, handling of deviations are addressed.
- On-site audits with documented proof of the measures defined in the audit report.
The availability of an up-to-date list of approved manufacturers and suppliers must be ensured so that orders can only be placed with approved suppliers and manufacturers.
5. Delivery
- Documentation of analysis data and rejections.
- Systematic monitoring of official inspection results.
6. Requalification:
- Renewed implementation of the qualification measures in accordance with the requirements of the risk management system.
7. Evaluation of changes:
- Changes on the part of the supplier are evaluated in relation to the risk as part of the documented change control procedure.
The structured and methodical implementation of supplier qualification ensures that all necessary qualifications are met, the quality of the materials or services to be supplied is assured and regulatory requirements are strictly adhered to. This promotes efficiency, transparency and compliance within the supply chain.
Is requalification possible?
Changes at the supplier or expiry of the validity of a qualification require re-qualification. A new version of the qualification is created and the relevant data is transferred. The qualification process is then carried out again.
For which time frame is a supplier qualified?
The validity date of a qualification is calculated based on the defined interval for the risk class.
How is a comprehensive supplier evaluation carried out in the context of GDP and GMP?
A comprehensive supplier evaluation in the context of GDP (Good Distribution Practice) and GMP (Good Manufacturing Practice) includes a systematic review of the supplier’s compliance with these regulatory requirements. This includes collecting and analysing relevant data to ensure that the supplier meets all necessary quality standards. Regular audits and the analysis of collected information ensure continuous supplier development and quality assurance.
What significance do data and information have in ensuring compliance in supplier qualification?
Data and information are of central importance in ensuring compliance in supplier qualification. Compliance with GDP and GMP guidelines is checked through the systematic collection and analysis of performance data. This information serves as a basis for the evaluation of suppliers and enables well-founded decisions for the continuous improvement of supplier development. Precise data usage helps to maintain the highest quality standards and ensure compliance.
Is qualification possible on the basis of material numbers?
In addition to the configured supplier types in the application, the individual material numbers can also be assigned to the qualification of a supplier.
Can the supply chain be mapped in the supplier qualification software?
In engamp | supplier qualification, material numbers, end products and locations can be assigned to a qualification to ensure a transparent supply chain.
Is it possible to store documents with the supplier qualification software?
The document dossier of a qualification is an essential part of engamp | supplier qualification. Depending on the risk class, a checklist is created with the necessary documents that must be requested, created and controlled.
Can audits be planned?
It will be possible to plan audits from a qualification. In addition to self-conducted audits with scheduling and selection of the audit team, third-party audits and written audits can also be planned.
Would you like to receive further information?
Take the opportunity to clarify your questions in a personal consultation and receive detailed information about the software – contact us now.
