QM system
Digital Life Sciences focuses on quality and ISO 9001

We are TÜV Rheinland tested and certified according to ISO 9001-2015.
This guarantees you that we meet the requirements of ISO 9001 in our quality management system and that we consistently meet the quality demands of our processes.
DIN EN ISO 9001:2015 is the most widely used and most important standard in quality management both nationally and internationally As the basis for the continuous improvement process, it serves primarily to meet customer requirements and to endeavour to meet customer expectations.
The certification consists of the demonstration of the practical application as well as a review of the documentation. With the successful certification, we demonstrate that we have mastered the high quality demands of our customers in the long term and that quality orientation in every sub-process determines our thinking and actions. Quality management is the driving force for the continuous increase in performance.
Our QM system
Our QM system consists, as most companies do, of software, hardware, processes, documents, records and people. As QM software, we use the products that we also sell to our customers. We are thus our own customer. In addition to document control and training management, we use the change control workflow for business process-relevant incidents.
Audits
As a supplier of software for companies in the GxP environment, conducting supplier audits is standard practice (e.g. in accordance with GAMP® 5).
Our products have also been successfully inspected by the regional councils / district governments and in TÜV audits in accordance with the guidelines of ISO standards 9001 and 13485.
Regular audits by our customers and interested parties also contribute to the continuous improvement of our process and product quality.
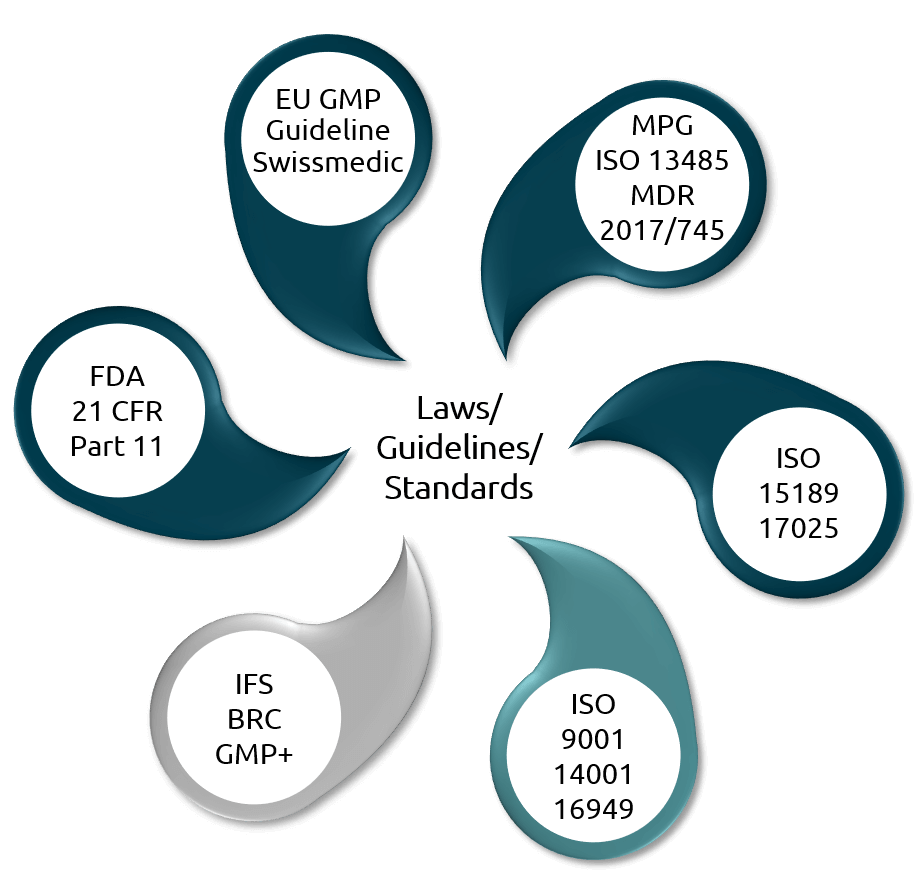
Regulations addressed
Our software solutions take into account the relevant laws, guidelines and standards in the regulated environment and have been developed specifically with this in mind. Our products have also been introduced and successfully validated by numerous companies in the life sciences sector.
Document Control
We use different document cycles in our company. In addition to the classical work and process instructions, also software specifications and quality assurance documents are managed, reviewed, approved and released.
All documents in our company are trained electronically. For this purpose, we have designated training coordinators who are given the task of determining the training relevance for their field of responsibility when new documents (first versions) are created. For follow-up versions, the system can thus deliver the relevant documents automatically.

Face-to-face trainings
We also plan our classroom training courses using the software we have developed for this purpose. We primarily train procedures and processes, which as part of our onboarding process, must be taught to the new employees. Data protection is another subject that we teach in face-to-face trainings. Although in the employment contracts, confidentiality and data protection declarations are signed, we consider it necessary that our employees are especially sensitized for this topic. In order to proof that trainings have been performed, we store the signed training sheets in the personnel files of our employees.
Process documentation
As a software and service company, we are TÜV Rheinland tested and certified according to ISO 9001:2015. As an information system for our employees, we document our business processes with the software we have developed for this purpose. In many companies this is a very complex task and all beginnings are difficult. Therefore, we have opted for a top-down approach and we have started with our main processes in the three fields management processes, core processes and support processes. These are continuously documented more granularly by including the corresponding sub-processes. All business processes we directly link with relevant work instructions and process instructions of our eQMS, but also with check lists, forms, white papers and encoded password lists of our eDMS.

Product development process
The product development process with respect to regulatory requirements demands a very high level of quality. In all areas of product development (development, documentation, quality assurance, product management), our QM system delivers the required work and process instructions. But also test design specifications, test reports, test plans, software specifications, test implementation reports, requirement documents and also technical and professional specifications are managed with our QM system and must pass a signature workflow which includes a content-related review and approval as well as a formal release and automatic implementation.
Would you like to learn more about our solutions?
Then look at our suitable solutions now.