Live webcast
QM processes
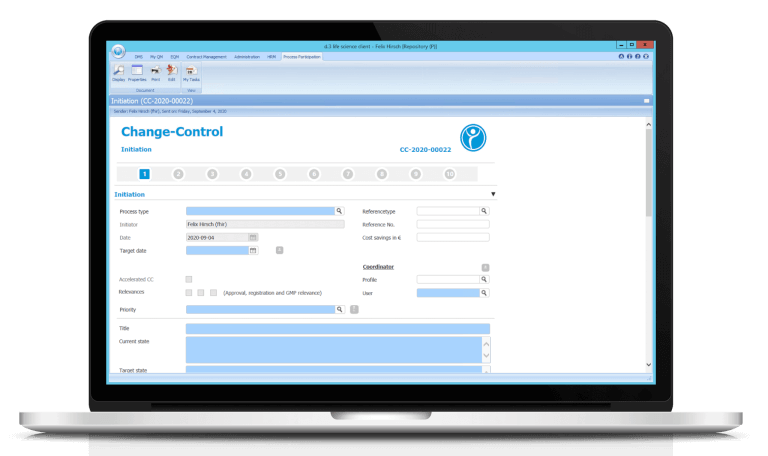
Manage QM processes successfully
- Learn how to digitally create, revise and sign work/process instructions (SOPs), process descriptions, test specifications, operating instructions, contracts or any other type of document.
- Learn how to manage and store a single or multi-level document circulation.
- Learn how the system automatically assigns appropriate “Periodic Review” tasks to the appropriate employees.
- Learn how the integrated reminder and escalation management signals when deadlines are exceeded.
- Learn how participation in the automatic document circulation is confirmed by an electronic GxP-compliant signature.
- Find out how traceability is ensured by means of an integrated audit trail for each document.
What you can expect
1. Presentation of Digital Life Sciences GmbH
2. Overview of solutions from Digital Life Sciences GmbH
3. Live presentation of a sample use case
4. Summary
5. References
Just a few steps to your goal:
1. Please use the form “Request Live Webcast” below
2. Fill in the contact fields.
3. We will approach you. By telephone or e‑mail.
Note on the live webcast: Specify your preferred date with one week’s notice.
Your contact person: Tim Dönnebrink
Request live webcast

Your WebCast consultant
Tim Dönnebrink is Head of Sales at Digital Life Sciences and looks after a large number of new and existing customers. In his role, he has specialized fully in presenting and advising on QM processes, among other things.
Request Webinar
oder
Request live webcast
Would you prefer to communicate with us by e‑mail? No worries!
Leave a message. We will get back to you!
