DOCUMENT CONTROL SOFTWARE
Efficient software for your Document Control
Digital Document Control ensures compliance with regulatory requirements and improves the transparency and traceability of your documentation-based processes.
Manage controlled documents efficiently and securely
Compliance with your regulatory requirements
Make business processes future-proof and digital

You are currently viewing a placeholder content from YouTube. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More Information» dls | document control «
What is Document Control?
Control your documents with the module "Document Control"
Our software solution “dls | document control” enables you to create, revise, and sign a wide range of document types completely digitally, including SOPs, work and process instructions, test specifications, operating instructions, guidelines, manufacturing documents, contracts, and more. The document circulation is configured flexibly so that different document circulations (single or multi-level) can be defined depending on the document template. The system automatically assigns “Periodic review” tasks to the respective employees. Missed deadlines and pending tasks are indicated by an integrated reminder and escalation management system. Participation in the automated approval process is confirmed by an electronic and GxP-compliant signature. Moreover, integrated audit trails increase the traceability and transparency of each document.
What are the benefits of Document Control?
How can you benefit from the Document Control software?
Digital Document Management
Digitally manage your specification documents (e.g. work and process instructions, test specifications, hygiene plans), forms, contracts, and other document types.
Completion at the touch of a button
Digitally handle the document distribution and the signature circulation at the touch of a button and focus on your core business.
Increased data security
Protect your data from unwanted interference by unauthorised users and from destructive forces.
Individual configuration
Let the modules be configured according to your needs and preferences to simplify the onboarding training of your colleagues.
Minimisation of process runtimes
Save time by minimising process runtimes for the creation, revision, release and distribution of your specification documents.
Reduction of resources
Reduce unnecessary printing and paper on your desk to protect the environment, avoid stress, and save money.
Central information platform
Use d.velop documents (formerly d.3ecm) as your central information platform to access your data at any time.
Constant availability
Access your data at any time and from anywhere.
How do I work with Document Control?
Get to know our Document Control software better
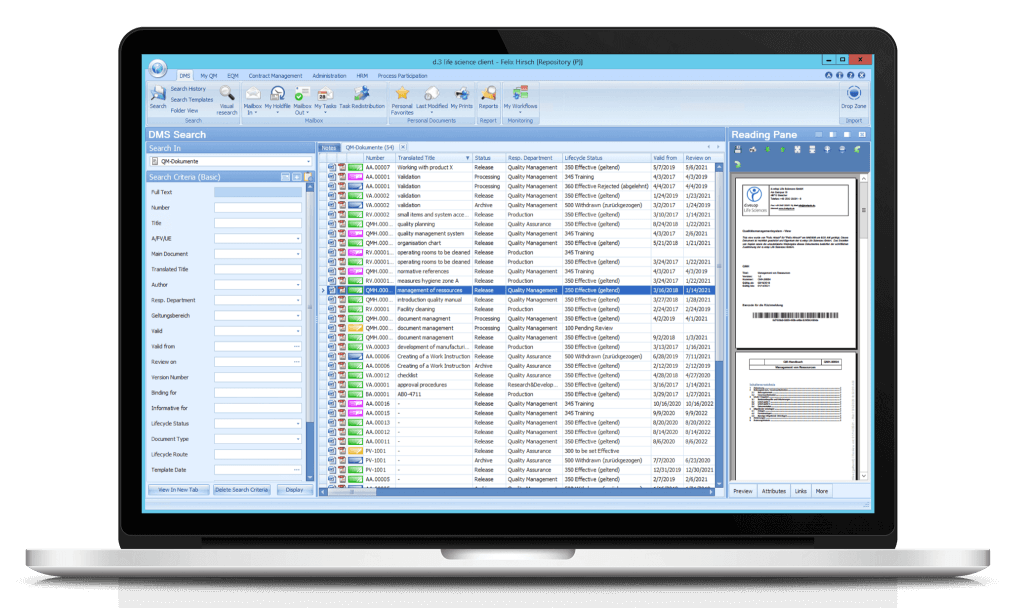
All documents and their properties that match your search criteria within the DMS search are displayed here.
The menu is opened via the dls | eQMS icon in the top left-hand corner. If you choose a menu or submenu item, the corresponding result list is displayed in the overview window.
Controlled and non-controlled documents as well as any document types and file formats can be stored by drag & drop via the Drop Zone.
For each document, a preview can be displayed in the flexibly customisable reading pane.
Depending on the user, licensed module, and authorisation, the following menu items are available in the dls | eQMS application window:
- DMS
- My QM
- Process Participation
- Administration
- Training Management
- Contract Management
- Personnel
The document status differentiates between:
- Processing (red)
- In circulation (yellow)
- Released (green)
- Withdrawn (blue)
- Training period (purple)
Documents can be found using various search criteria and attributes. The search result is displayed in a result list on the right side in a tab. Of course, a full-text search as well as a search via visual elements is also possible.
What does Document Control look like live?
Would you like a live insight into Document Control software?
Get a personal live insight into the many possibilities of Document Control in just 45 minutes using an illustrative use case. Find out how you can use Digital Life Sciences solutions to electronically create, revise, and digitally sign your work and process instructions (SOPs), process descriptions, test specifications, operating instructions, contracts, or any other type of document.
What functions does Document Control have?
Some features of the Document Control
- Creation of controlled documents within the system based on approved templates
- The templates can also be controlled documents.
- Management of specification documents and forms
- Digital document signature using integrated GxP-compliant signature - entirely software-based
- Full-text search based on the content of the documents and their attributes (metadata)
- Individual and dynamic compulsory watermark
- Easy creation of report lists
- Controlled and logged printing
- Comparison of different document versions and display of the differences
- Integrated archiving using the ECM d.velop documents (formerly d.3ecm)
- Windows and web frontend
- Periodic review
Which regulations apply to Document Control?
Which regulations must be observed for controlled documents?
ISO 9001:2015, Chapter 7.5
ISO 13485:2016, Chapter 4
EU-GMP guideline, Annex 11
WHO Guidelines on Good Practices in Data and Record Management
EFG-Votum V11002: Requirements regarding the electronic data storage
EFG-Votum V11003: Requirements regarding electronic signatures and initials
Document Control White Paper
White Paper: Digital Document Control for your company
Read our free White Paper to find out how you can optimise your processes and easily meet regulatory requirements with digital Document Control.
You will receive further information by e-mail. Get additional information on data protection here.

“Years ago, we used to track the status of documents such as work instructions, employee's proofs of qualification, and batch reports using an Access database or on paper. However, it was tedious to keep the documents up to date and to manage them in an audit-compliant manner. In 2012, we switched to the digital Document Control by Digital Life Sciences, which was a quantum leap. Today we can create, find, edit and agree upon documents much faster. This saves working time and costs."

Which software can be used together with Document Control?
You might also like...
Document Control is a component of the Digital Life Sciences solution suite. Each product is powerful on its own, but when used together they are even better.

Training Management software
Extend the module “Document Control” to actively plan and log your employees' qualifications with our training management software.

E-learning software
Use the new “E-learning” software to train your employees digitally. Create e-learnings according to your wishes using Microsoft PowerPoint or integrate existing presentations.
QM processes (Complaint | DC | CAPA | CC)
Digitalise your ISO processes. Control your production-related QM processes using digital workflows.
Document Control FAQ
Frequently asked questions (FAQs) about Document Control
- Can SOPs be divided into different categories?
For the management of QM documents, the dls | eQMS can manage the three document categories ‘QM manual’, ‘Process instructions’ and ‘Work instructions’. These three document categories reproduce different hierarchical levels of the QM documentation. The QM manual includes universally valid documents such as policies, for example, and refers to the other two underlying hierarchy types for a detailed definition of operating instructions.
- Can a numbering scheme for SOPs be configured?
By means of a numbering scheme, a unique number can be assigned to each document in the dls | eQMS. Hierarchical numbering is also available here, which has an impact on the release processes. For example, an attachment created with a hierarchical numbering system can only be released if the superordinate document is released.
- How are further applicable documents and attachments managed?
One or more further applicable documents and/or attachments can be assigned to a controlled document. They are listed in the document properties and in the preview and can also be accessed directly here.
You can configure that the author of a document receives a notification when a further applicable document or attachment is released in a new version.
- Can the document circulation be configured?
Document circulations (e.g. review, approval, release, training, setting effective) can be flexibly configured and adapted to your company requirements. The document circulation is linked to the document template; thus, a different document circulation could be defined for each template.
- Can specifications be set regarding the selection of persons for the circulation steps?
You can determine whether and which employees and/or employee profiles can be selected in each individual step. You can also determine whether a minimum or maximum number of people are involved in a step and specify a particular person/profile or make it mandatory.
- Are released QM documents automatically set effective?
Setting a document effective is always the last step in a document circulation and usually takes place automatically at the end of the scheduled training period. However, it is also possible to set documents manually effective.
- Can the document circulation be supplemented by a periodic review?
In a document circulation, you can decide if you want to have a periodic review task sent. If a periodic review is planned, the releaser receives a date proposal based on the configuration (e.g. 2 years after the document has been set effective) and a periodic review task is sent appropriately in advance.
The task for the periodic review can be sent to different persons or activity profiles (e.g. reviewers, approvers, releasers).
- How is the signature implemented within the document circulation?
An electronic signature is implemented for all actions requiring signatures (document circulation, read-and-understood tasks etc.). It meets the requirements of the FDA and the GMP guidelines and replaces the personal signature on a printed document. This feature enables responsible persons to provide the necessary signatures even at a distance. Each signature is recorded in the audit trail and printed on an additional page with the document.
- Are all versions of a controlled document managed?
Controlled documents are managed in a minor and major versioning. There may be multiple editing versions if the review/approval/release has been rejected in the document circulation. The author creates a new processing version and restarts the circulation. All versions of a controlled document can be viewed by authorised users. A direct document comparison of two versions is also available here.
- Can a document be edited by several employees?
A document is assigned to an author to whom editing is reserved. However, authorship can be transferred to another employee so that they can add further content if necessary.
- How do employees receive QM documents?
Independently of the additional features in the ‘training management’, you can store a document distribution list on the basis of which the list ‘Binding for’ for a document is created. If document distribution is used, a first distribution list is specified in the document circulation. In the first distribution list, those employees are selected who will receive the task for the read-and-understood training after the document is released. In connection with the processing of this task, a sub-distribution option is available. The employee can distribute the document to other employees in connection with the read-and-understood training.
- Are printouts of a controlled document documented?
With appropriate authorisation, an employee can print controlled documents. During controlled printing, the employee selects the extent to which the document should be printed (additional pages with audit trail information, electronic signatures etc.). A printing reason must be specified and the printout is logged in the properties.
If the printout is reported as returned/destroyed, this is noted in the system using the function ‘Confirmation of printout return’.
- How can document control improve the lifecycle of documents and records in a company?
Document Control improves the lifecycle of documents and records by ensuring that all documents and information are controlled systematically and efficiently. A DMS supports these processes by organising the entire lifecycle from the creation and archiving to the disposal of documents and records. This leads to improved documentation and seamless integration into the company’s quality management.
- Why is Document Control important for managing information and changes in a company?
Document Control is essential for the management of information and changes, as it ensures that all documents and information are correctly documented and monitored. With a DMS, changes and important records can be tracked seamlessly, which increases the quality of the information. This is crucial for reliable quality management and helps the company to ensure a high level of accuracy and consistency in its processes.
- How does Document Control contribute to the effective archiving and management of documents and records?
Document Control contributes to the effective archiving and management of documents and records by implementing processes that ensure the correct handling and storage of all documents. A DMS enables companies to manage documents and information centrally, which simplifies archiving and management. This supports documentation and ensures that all documents are correctly controlled and available throughout their entire lifecycle.
- What advantages does a document management system (DMS) offer in the context of Document Control and process optimisation?
A DMS offers numerous advantages in the context of Document Control by improving process optimisation and the management of documents and information. Electronic archiving and the central management of records enable companies to make their documentation more efficient and transparent. A DMS supports traceability and quality management by organising and making accessible all changes and important information.
- To what extent can efficient document management improve knowledge and information within the company?
Efficient Document Control can significantly improve knowledge and information within the company by ensuring that all relevant documents and information are correctly controlled and documented. A DMS facilitates access to important records and information and stores changes in a traceable manner. This promotes internal knowledge, optimises processes and improves quality management, as all employees always have access to up-to-date and accurate information.
- What role does Document Control play in the risk management process and how is information controlled?
Document Control plays a key role in the risk management process, as it ensures that all relevant information is systematically controlled and centrally administered. Thanks to a clearly structured process, all documented risks are precisely recorded and crucial information is made available at all times. This organised management of information helps to identify potential risks at an early stage and initiate appropriate measures, thereby optimising overall risk management.
For the management of QM documents, the dls | eQMS can manage the three document categories ‘QM manual’, ‘Process instructions’ and ‘Work instructions’. These three document categories reproduce different hierarchical levels of the QM documentation. The QM manual includes universally valid documents such as policies, for example, and refers to the other two underlying hierarchy types for a detailed definition of operating instructions.
Document circulations (e.g. review, approval, release, training, setting effective) can be flexibly configured and adapted to your company requirements. The document circulation is linked to the document template; thus, a different document circulation could be defined for each template.
You can determine whether and which employees and/or employee profiles can be selected in each individual step. You can also determine whether a minimum or maximum number of people are involved in a step and specify a particular person/profile or make it mandatory.
Setting a document effective is always the last step in a document circulation and usually takes place automatically at the end of the scheduled training period. However, it is also possible to set documents manually effective.
In a document circulation, you can decide if you want to have a periodic review task sent. If a periodic review is planned, the releaser receives a date proposal based on the configuration (e.g. 2 years after the document has been set effective) and a periodic review task is sent appropriately in advance.
The task for the periodic review can be sent to different persons or activity profiles (e.g. reviewers, approvers, releasers).
Independently of the additional features in the ‘Training Management’, you can store a document distribution list on the basis of which the ‘Binding for list’ for a document is created. If document distribution is used, a first distribution list is specified in the document circulation. In the first distribution list, those employees are selected who will receive the task for the read-and-understood training after the document is released. In connection with the processing of this task, a sub-distribution option is available. The employee can distribute the document to other employees in connection with the read-and-understood training.
Document Control improves the lifecycle of documents and records by ensuring that all documents and information are controlled systematically and efficiently. A DMS supports these processes by organising the entire lifecycle from the creation and archiving to the disposal of documents and records. This leads to improved documentation and seamless integration into the company’s quality management.
Document Control is essential for the management of information and changes, as it ensures that all documents and information are correctly documented and monitored. With a DMS, changes and important records can be tracked seamlessly, which increases the quality of the information. This is crucial for reliable quality management and helps the company to ensure a high level of accuracy and consistency in its processes.
Document Control contributes to the effective archiving and management of documents and records by implementing processes that ensure the correct handling and storage of all documents. A DMS enables companies to manage documents and information centrally, which simplifies archiving and management. This supports documentation and ensures that all documents are correctly controlled and available throughout their entire lifecycle.
A document is assigned to an author to whom editing is reserved. However, authorship can be transferred to another employee so that they can add further content if necessary.
By means of a numbering scheme, a unique number can be assigned to each document in the dls | eQMS. Hierarchical numbering is also available here, which has an impact on the release processes. For example, an attachment created with a hierarchical numbering system can only be released if the superordinate document is released.
One or more further applicable documents and/or attachments can be assigned to a controlled document. They are listed in the document properties and in the preview and can also be accessed directly here.
You can configure that the author of a document receives a notification when a further applicable document or attachment is released in a new version.
With appropriate authorisation, an employee can print controlled documents. During controlled printing, the employee selects the extent to which the document should be printed (additional pages with audit trail information, electronic signatures etc.). A printing reason must be specified and the printout is logged in the properties.
If the printout is reported as returned/destroyed, this is noted in the system using the function ‘Confirmation of printout return’.
An electronic signature is implemented for all actions requiring signatures (document circulation, read-and-understood tasks etc.). It meets the requirements of the FDA and the GMP guidelines and replaces the personal signature on a printed document. This feature enables responsible persons to provide the necessary signatures even at a distance. Each signature is recorded in the audit trail and printed on an additional page with the document.
Controlled documents are managed in a minor and major versioning. There may be multiple editing versions if the review/approval/release has been rejected in the document circulation. The author creates a new processing version and restarts the circulation. All versions of a controlled document can be viewed by authorised users. A direct document comparison of two versions is also available here.
A DMS offers numerous advantages in the context of Document Control by improving process optimisation and the management of documents and information. Electronic archiving and the central management of records enable companies to make their documentation more efficient and transparent. A DMS supports traceability and quality management by organising and making accessible all changes and important information.
Efficient Document Control can significantly improve knowledge and information within the company by ensuring that all relevant documents and information are correctly controlled and documented. A DMS facilitates access to important records and information and stores changes in a traceable manner. This promotes internal knowledge, optimises processes and improves quality management, as all employees always have access to up-to-date and accurate information.
Document Control plays a key role in the risk management process, as it ensures that all relevant information is systematically controlled and centrally administered. Thanks to a clearly structured process, all documented risks are precisely recorded and crucial information is made available at all times. This organised management of information helps to identify potential risks at an early stage and initiate appropriate measures, thereby optimising overall risk management.
Quick contact
Do you have a question about Document Control?
Our sales team will help you promptly and gladly.
Attention: Form is
currently not supported by:
⛔ iPhones with iOS version <14

