Definition of the term ("What is a SOP?")
Standard operating procedures (SOPs) are written documents that provide detailed instructions for specific work processes in a company or organization. These instructions are formulated to help employees perform tasks in a consistent and efficient manner. SOPs are widely used in various industries, including manufacturing, healthcare, IT, and many others. The use of SOPs ensures that all procedures are carried out consistently and comply with current standards.
The significance of SOPs
SOPs play a critical role in ensuring that a company operates smoothly. Here are some important reasons why they are of great significance:
- Consistency and quality assurance: Using SOPs ensures that all employees follow the same standards and procedures. This leads to consistent quality in the services or products provided.
- Increasing efficiency: SOPs define clear steps and responsibilities, thus, making work processes more efficient. Employees know exactly what is expected of them, thus, saving time and resources.
- Training and onboarding training: SOPs can be valuable training tools for new employees. They enable faster onboarding training and minimise the risk of errors.
- Compliance and security: In some industries, SOPs are required by law to ensure compliance with regulations and safety standards.
How do you create effective SOPs?
Creating effective SOPs requires care and precision. Here’s a step-by-step guide to creating high-quality SOPs:
- Step 1: Identify the process
Select the work process for which you want to create an SOP. Clearly define what this process includes. - Step 2: Gather information
Talk to employees who already know the process to gather all relevant information. Note down all the steps and details. - Step 3: Formulate clear instructions
Write the instructions in simple, understandable language. Avoid jargon and complicated sentences. - Step 4: Visualise the process
Use diagrams, flowcharts, or graphs to visually represent the process. This facilitates understanding. - Step 5: Training of the employees
Ensure that all affected employees thoroughly understand the SOPs. This requires training and educational materials to ensure everyone is on the same page. - Step 6: Test, monitor, and update the SOP
Have selected employees perform the process using the SOP and accept feedback. SOPs should not be set in stone. It is important to review them regularly and update them as needed to ensure they meet changing requirements and best practices.
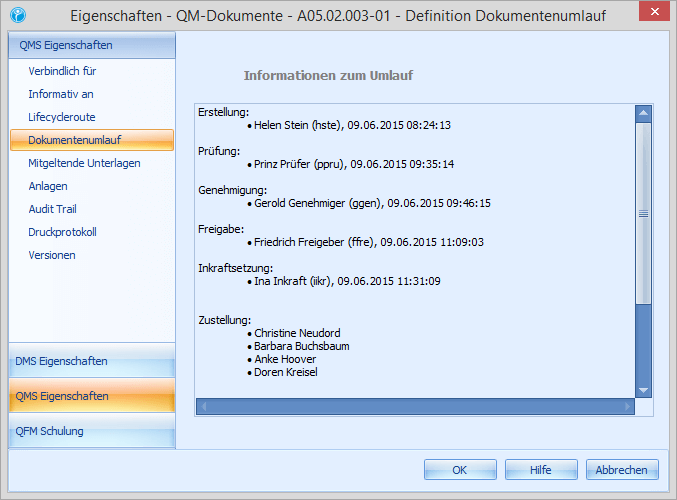
An SOP is documented in a handbook. It contains a distinct ID, a version number, a date or period of validity and the names and signatures of the creator, approver and releaser. This information ensures a comprehensive traceability of a document. Moreover, it must be recorded that the employees of the organisation were informed of the content of an SOP and must be notified in case of a change to an SOP.
Discover the right software for your SOP management
Whether work / process instructions (SOPs), process descriptions, test specifications, operating instructions, contracts, or any other types of documents — you can create, revise and sign them digitally with our module “document control”. Compliant with ISO standards, EU-GMP, FDA CFR & more.

SOPs in practice
A good example of the application of SOPs is a medical laboratory. Here, SOPs are essential to ensure that tests are performed correctly. Each step, from sample management to analysis, is precisely defined to ensure accuracy and safety.
In which sectors/industries is it required?
They are a requirement for the official approval, especially in the pharmaceutical industry. This especially affects the certification of products and services. Since this is a regulatory approval, the authority also checks the content. It is investigated whether there are violations of corresponding procedures. If this is the case, this can result in various consequences down to the prohibition of selling product or services.
What are the advantages of SOPs?
Due to the possible authoritative sanctions, standard operating procedures are stronger than ordinary work instructions describing the procedures within an organisation etc. Other advantages of electronically managed SOPs (made possible by the document control module) include:
- Verification and release processes controllable with workflows
- Comprehensive document management incl. versioning
- Compliance with all requirements/regulations
- Electronic management of your specifications (e.g. work /process instructions, test specifications, hygiene plans, operating instructions), form sheets, contracts and other document types
- Reduction of process runtimes for creation, revision, release and distribution of specifications
- Easy distribution of documents and use of the signature circulation
- Reduction of unnecessary printouts and paper
Conclusion
Standard operating procedures (SOP) are a powerful tool for optimising work processes. They increase efficiency, quality and safety in companies and organisations. Creating and maintaining SOPs takes time and commitment, but pays off in the form of smooth processes and satisfied customers.
Start your digital transformation with our powerful, modular SOP management software solution
Frequently asked questions (FAQs) - Standard Operating Procedure (SOP)
Why are SOPs important in companies?
Standard operating procedures (SOPs) are crucial for quality assurance, efficiency and safety in companies. They ensure consistent standards, make it easier to train new employees and help with compliance, especially in regulated sectors such as the life sciences industry. SOPs promote transparent processes and increase competitiveness through operational excellence.
Can I create SOPs for each business area?
The creation of standard operating procedures (SOPs) is extremely valuable in various industries and promotes the efficiency, uniformity and quality assurance of operational processes. Especially in regulated industries such as pharmaceuticals and medical technology, SOPs are essential for standardising internal processes. They are also important in other areas such as financial services or production to ensure operational efficiency. Specific SOPs for different business areas promote transparency and support the digital transformation. The creation of SOPs is therefore crucial for process optimisation and a company’s competitive advantage.
Is there software that is helpful in creating SOPs?
The document control software solution from Digital Life Sciences GmbH enables the efficient creation and management of standard operating procedures in regulated sectors such as the life sciences industry. It optimises the entire life cycle of SOPs, from creation and approval to audit-compliant archiving. This technology keeps documentation up to date and compliant with regulatory requirements, which increases efficiency and reduces the risk of non-compliance. The digital transformation supports transparency and traceability of processes for successful operational management.
Where can I find more resources for creating SOPs?
To create effective standard operating procedures (SOPs), reference books and online courses can be valuable resources. These offer theoretical knowledge and best practices. In addition, trade journals provide the latest findings and innovations in quality management to ensure that SOPs meet the latest requirements. By using these resources, you strengthen your role as a quality management representative and contribute to efficiency and transparency in your company.
What is a Standard Operating Procedure (SOP)?
The creation and implementation of Standard Operating Procedures (SOPs) is crucial for an effective quality management system. SOPs define precisely how tasks should be carried out systematically and uniformly in order to achieve consistent results. The use of SOPs minimises errors, increases efficiency and ensures uniform quality. They are essential for compliance with regulatory requirements and promote a culture of continuous improvement in companies.
Why are SOPs important in quality management?
Standard operating procedures (SOPs) are essential in quality management in order to ensure uniform standards and comply with legal requirements in the life science industry. SOPs provide clear instructions for quality assurance in production, minimize deviations and strengthen confidence in product quality. SOPs give employees the knowledge and skills they need to reduce errors and make processes more efficient.
How can an SOP be written correctly?
To write an SOP correctly, choose a clear and structured approach. A well-formulated SOP serves to document processes, efficiency and compliance. Start with a precise explanation of the SOP and clearly define its objective. Organise the document into comprehensible steps. Clear and precise wording is essential to avoid misunderstandings. An SOP template can be helpful in order to insert important elements consistently. Pay attention to responsibilities, resources and specific requirements.
How often should SOPs be updated?
The regular review and adaptation of standard operating procedures (SOPs) is essential in quality management in the life science industry. Updates ensure compliance with current requirements and best practices, support the introduction of new information and technologies and ensure compliance with FDA 21 CFR Part 11 and EU GMP. Internal feedback from quality management, production, and IT is crucial for practicable SOPs. Continuous updating strengthens quality, trust and transparency within the company.
What tasks does an SOP include and how do I process them efficiently?
A standard operating procedure (SOP) comprises a large number of tasks aimed at standardising processes and procedures within a company. Typical tasks include the detailed documentation of work processes, safety protocols and quality assurance measures. In order to process these tasks efficiently, it is advisable to define clearly defined steps and responsibilities in the SOP. A well-structured SOP facilitates the processing of complex documentation by providing clear instructions for handling new procedures and implementing them in existing processes.
How does an SOP contribute to quality assurance when introducing new procedures?
A standard operating procedure (SOP) plays a crucial role in quality assurance, especially when introducing new procedures. The detailed documentation of the new processes and steps in the SOP ensures that all employees work on the same tasks in a consistent manner. This minimises errors and ensures that the new procedures are carried out in accordance with the defined standards. The SOP serves as a reference document and provides clear instructions that support the efficient implementation and monitoring of new process steps, thereby contributing to overall efficiency and quality assurance within the company.
Practical example of SOP management in the company:
Our company example is Mustermann GmbH. The last internal audit in the production department resulted in necessary changes and improvements in the maintenance process. In this case Erwin Ersteller is responsible for this process. The company’s document control system specifies exactly which steps the document must go through before the new version is published. The description of the maintenance process is defined as a 4-level document and therefore goes through the following steps:
1. Create/revise 2. Review 3. Approve 4. Release 5. Training 6. Setting effective
(Click on the process steps below for more information)

Our company example is Mustermann GmbH. The last internal audit in the production department resulted in necessary changes and improvements in the maintenance process. In this case Erwin Ersteller is responsible for this process. The company's document control system specifies exactly which steps the document must go through before the new version is published. The description of the maintenance process is defined as a 4-level document and therefore goes through the following steps:
- Create/revise
- Review
- Approve
- Release
- Train
- Enact
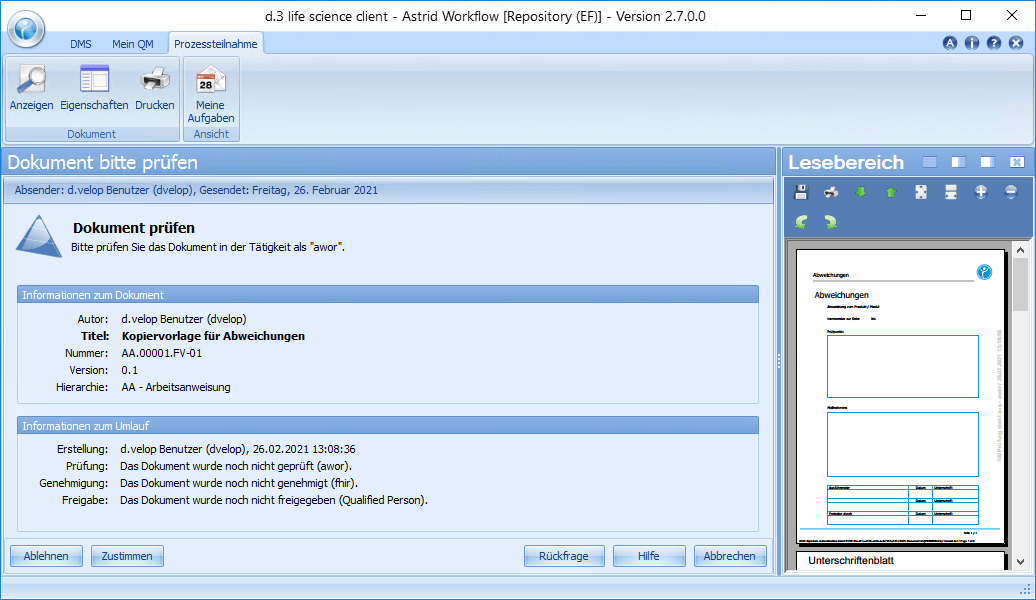
Erwin Ersteller creates a new document based on a template or an existing document, or creates a new version of an existing SOP, for example. Of course, he can also involve several editors in the creation process. After creating or modifying the document, Erwin Ersteller sets the document circulation (review, approval and release) and finishes the editing. Using the workflow system, the document is automatically forwarded to the responsible reviewer (one or more), who receives a corresponding task.
In this step, the content check takes place. Paul Prüfer, the responsible reviewer of the document, opens his task and studies the contents of the document sent to him. He has the possibility to approve the document and thus pass it on to the next steering step or to reject the document, to add comments/comments/wishes for changes and to send it back to Erwin Ersteller. In addition, enquiries can be placed about the document. If the document is approved, it will be forwarded to Adam Approver.
Learn how the process can be implemented digitally:
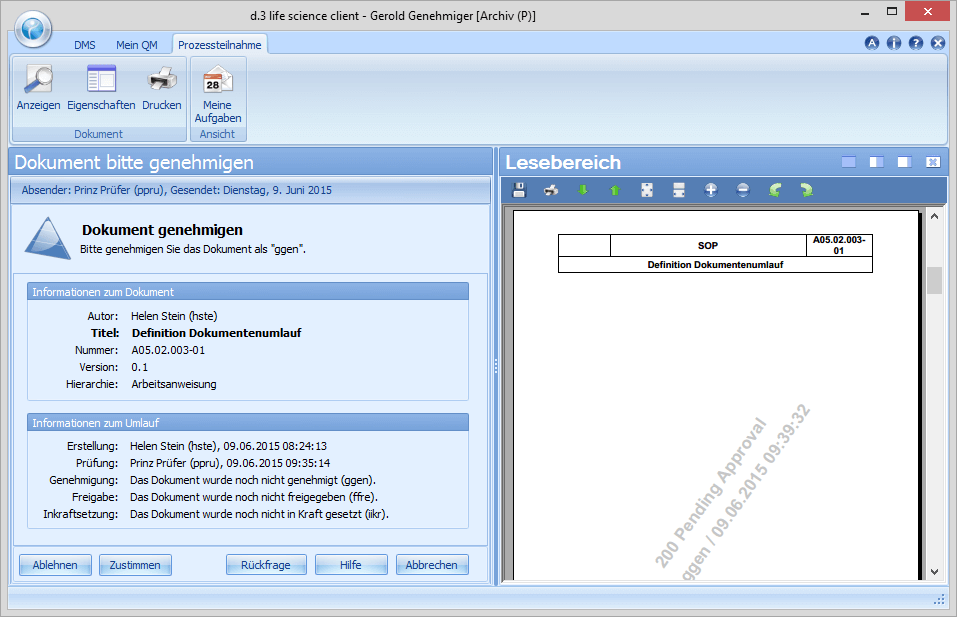
In the course of the approval Gerold Genehmiger has the same options/functions as Paul Prüfer. If he discovers formal errors or if generally necessary corrections need to be made, he can add his comments and send the document back to the creator. If Adam Approver approves the document, it is sent to Richard Releaser for release.
Learn how the process can be implemented digitally:
Learn how the process can be implemented digitally:
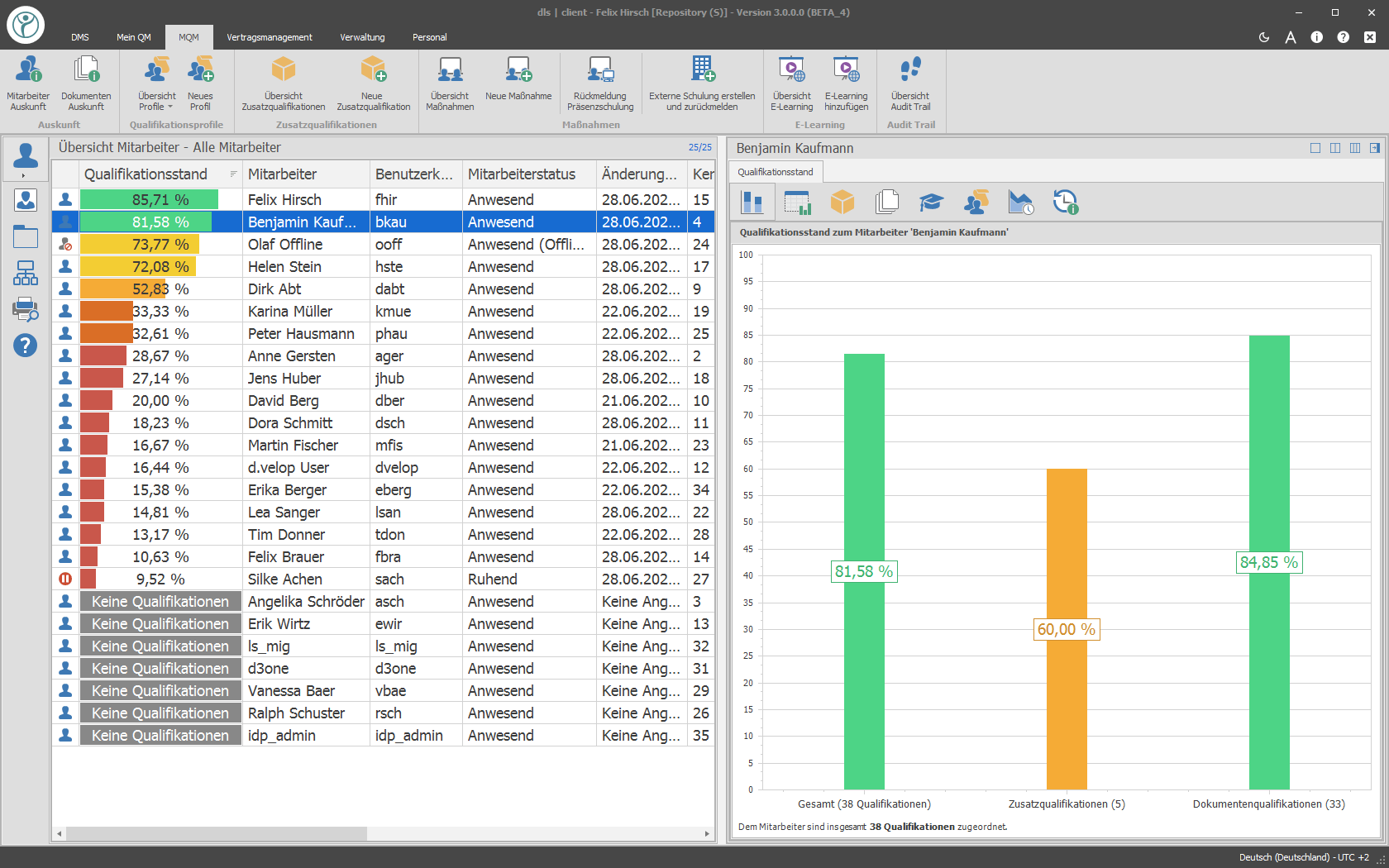
With the release of a new document or revision, a training requirement is generated. All employees who need to know this document will receive a training task within the system. The automatically formed qualification matrix shows you at any time at the push of a button which training level your employees have. In the area of training, a variety of training types (reading training, reading training with electronic exam, e-learning, face-to-face training, etc.) are available.
Learn how the process can be implemented digitally:
After the defined training phase, the document automatically takes effect (unless configured otherwise). The document remains valid until a new version is created and the document circulation starts again. Shortly before reaching (time period individually selectable) the specified validity date of the document, a task for periodic review is automatically sent, usually to the creator. The recipient can, if no changes are necessary, extend the runtime of the document by another cycle. If changes are necessary, a new version is created.
Learn how the process can be implemented digitally:

Our company example is Mustermann GmbH. The last internal audit in the production department resulted in necessary changes and improvements in the maintenance process. In this case Erwin Ersteller is responsible for this process. The company’s document control system specifies exactly which steps the document must go through before the new version is published. The description of the maintenance process is defined as a 4-level document and therefore goes through the following steps:
- Create/revise
- Review
- Approve
- Release
- Train
- Enact
Erwin Ersteller creates a new document based on a template or an existing document, or creates a new version of an existing SOP, for example. Of course, he can also involve several editors in the creation process. After creating or modifying the document, Erwin Ersteller sets the document circulation (review, approval and release) and finishes the editing. Using the workflow system, the document is automatically forwarded to the responsible reviewer (one or more), who receives a corresponding task.
In this step, the content check takes place. Paul Prüfer, the responsible reviewer of the document, opens his task and studies the contents of the document sent to him. He has the possibility to approve the document and thus pass it on to the next steering step or to reject the document, to add comments/comments/wishes for changes and to send it back to Erwin Ersteller. In addition, enquiries can be placed about the document. If the document is approved it will be forwarded to Adam Approver.
Learn how the process can be implemented digitally:
In the course of the approval Gerold Genehmiger has the same options/functions as Paul Prüfer. If he discovers formal errors or generally necessary corrections need to be made, he can add his comments and send the document back to the creator. If Adam Approver approves the document it will be sent to Richard Releaser to be released.
Learn how the process can be implemented digitally:
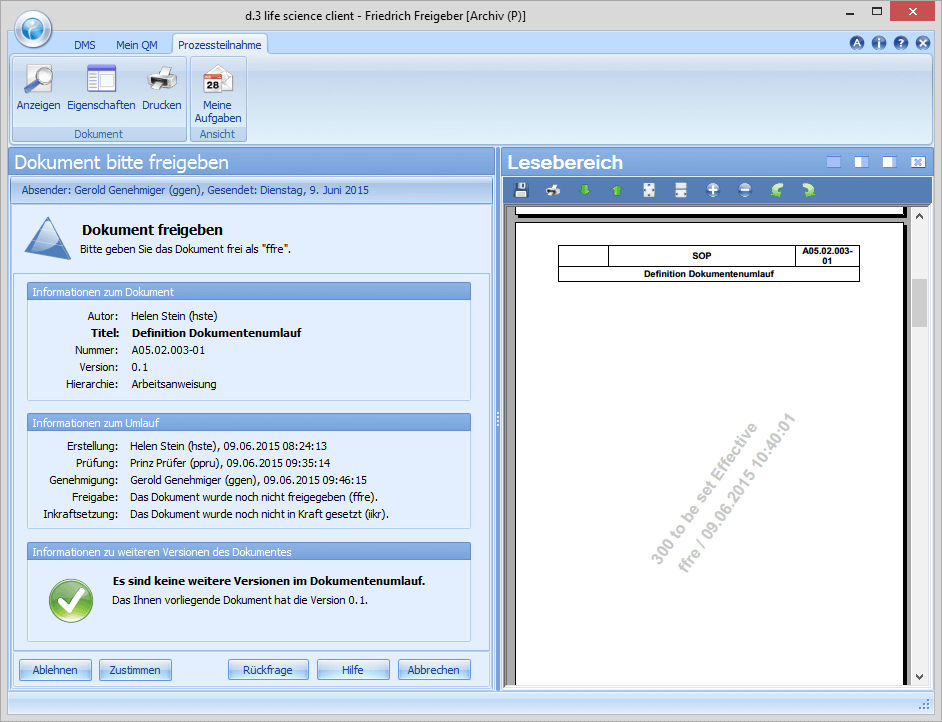
The department head Friedrich Freigeber is responsible for the release of the document or the new revision. If there are no objections, he confirms the release by means of a digital signature and completes the workflow for the document as such. In the release, Friedrich Freigeber can define validity dates such as valid from, valid until (periodic review) with reference to the document and, among other things, define training coordinators.
Learn how the process can be implemented digitally:
With the release of a new document or revision, a training requirement is generated. All employees who need to know this document will receive a training task within the system. The automatically formed qualification matrix shows you at any time at the push of a button which training level your employees have. In the area of training, a variety of training types (reading training, reading training with electronic exam, e-learning, face-to-face training, etc.) are available.
Learn how the process can be implemented digitally:
After the defined training phase, the document is automatically set effective (unless configured otherwise). The document remains valid until a new version is created and the document circulation starts again. Shortly before reaching (time period individually selectable) the specified validity date of the document, a task for periodic review is automatically sent, usually to the creator. The recipient can, if no changes are necessary, extend the runtime of the document by another cycle. If changes are necessary, a new version is created.
Learn how the process can be implemented digitally: