Validation as a Service (VaaS) – what exactly is that?
Validation as a Service (VaaS) refers to the outsourcing of the continuous validation of computerised systems (Computer System Validation, CSV) to specialised service providers. In regulated industries such as pharmaceuticals, medical technology, food, and cosmetics, VaaS ensures that software, systems, and processes operate reliably, securely, and in compliance with regulations at all times. Validation provides objective evidence that the specific requirements arising from the intended use and user needs are consistently met over time.
The goal is to identify and minimise risks to product quality, patient safety, and data integrity at an early stage. Validation creates transparency and control, ensuring that only systems with acceptable residual risks are used.
Compliance with relevant standards and guidelines – such as GxP, ISO, and FDA – is recognised by notified bodies as evidence that legal requirements have been met.
Problem: Time-consuming validation projects delay software updates
Many companies face the challenge that validation projects are time-consuming and resource-intensive, which delays software updates. Although manufacturers often provide generic documentation and standardised tests, these are not sufficient for complete validation in the life sciences environment. Because application-specific assessment is crucial:
- How is the software used in the specific process?
- What are the risks to product quality, patient safety, or data integrity?
Quality management software, for example, can be delivered by the manufacturer with generic functions such as document control, audit trail, and user management. These functions have already been tested and documented. These preparatory steps by the manufacturer make implementation significantly easier. However, an application-specific validation is still required before the software can be used in GxP-critical processes. The following questions, however, cannot be answered by the software manufacturer:
- Configuration: Which workflows are used? Which document types are GxP-relevant?
- User roles: Who is authorised to release, modify, or delete – and how is this documented?
- Interfaces: How is the application connected to other systems (e.g., LIMS, ERP)?
- Data integrity: How is it ensured that no critical data is lost or manipulated?
These aspects are individual and application-specific – therefore, a blanket validation by the manufacturer is not possible. The responsibility for full validation rests with the user, as they alone understand how the system is actually used in the process.
Furthermore, validation is not a one-time process: every change to the system, new regulatory requirements, or cybersecurity risks require continuous review and adjustment.
In many companies, validation is carried “on the side” – in addition to regular day-to-day operations. Limited resources and time pressure can lead to bottlenecks, delays in software updates, and risks to quality and compliance. Validation projects tie up resources and delay updates – especially in the case of complex, individually configured systems.
Benefits of Validation as a Service
This is precisely where Validation as a Service (VaaS) comes in. A specialised service provider takes care of the continuous validation of computerised systems (CSV) – efficiently, scalably, and in compliance with regulations.
Additional advantages include:
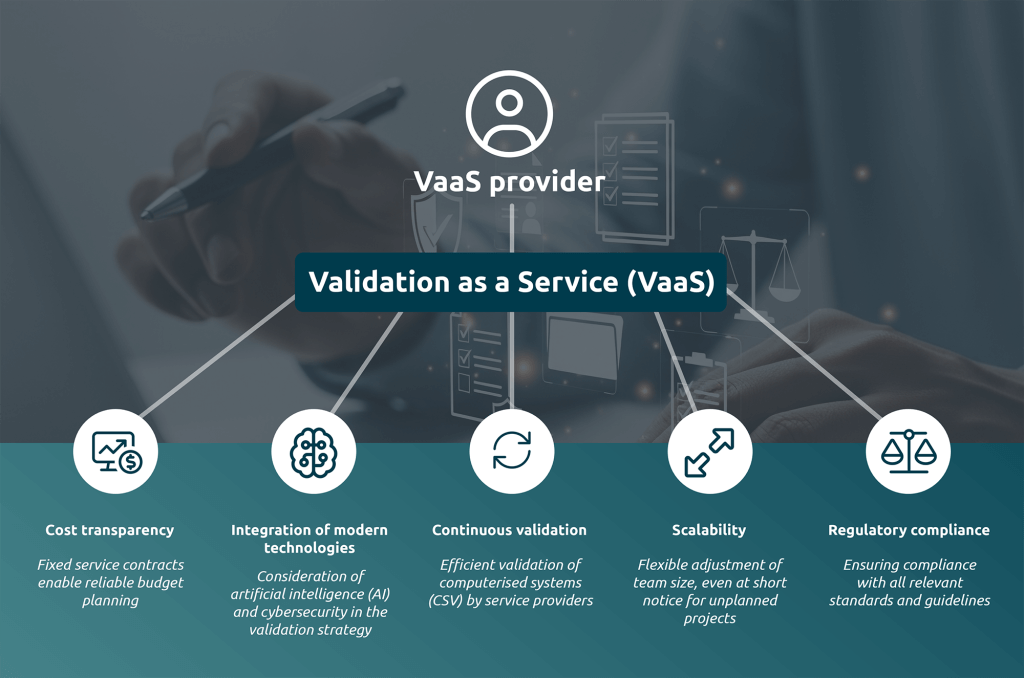
- Resource relief: IT and quality management teams are sustainably relieved of workload
- Cost transparency: Fixed service contracts enable reliable budget planning
- Scalability: Flexible adjustment of team size, even at short notice for unplanned projects
- Regulatory compliance: Ensuring compliance with all relevant standards and guidelines
- Integration of modern technologies: Consideration of artificial intelligence (AI) and cybersecurity in the validation strategy
- Central point of contact: Unified support for all validation-related systems and processes
By outsourcing to experienced CSV specialists, companies gain security, transparency, and freedom to focus on strategic tasks.
How does Validation as a Service work in practice?
VaaS service providers develop an individual, end-to-end validation strategy for single systems, system networks, or entire departments. They accompany all software releases, perform standard-compliant validations, and document them according to customer-specific specifications.
Support is provided on an ongoing basis to ensure that the validated state is maintained at all times. This helps prevent malfunctions and downtime in productive environments – especially in critical processes and for sensitive products.
Use cases and examples
An example of VaaS is the management of a proprietary, complex logistics system network with several connected systems.
Service providers develop a validation strategy for this network, carry out the validations, and document the activities and results in accordance with standards and customer specifications. They ensure that these systems remain valid over time, regardless of changes.
As the central point of contact for all validation-related matters within the system network, they can support regular validation tasks while also acting as the main point of contact for cross-system release planning. Through ongoing support of the system network, they gain knowledge about the state and planned changes to the individual systems, as well as the interdependencies between the systems. This allows them to address potential issues within the system network early in the release planning process – for instance, regarding necessary downtimes for implementing updates – and help resolve them in advance. With their understanding of how systems are interconnected, they can also support the assessment of whether a change in one system affects others.
When validation is carried out solely on a project basis, prioritisation often becomes challenging – critical systems with higher risk or regulatory relevance may be overlooked. Moreover, there is a risk that cross-system interdependencies may not be sufficiently taken into account. Validation as a Service is ideal for establishing validation as a cross-functional task for all relevant software solutions. This creates an overall view that enables a strategic and efficient approach. The validation partner has detailed knowledge of systems and their use and can therefore perform targeted, consistent, and forward-looking validation.
When is Validation as a Service particularly worthwhile?
VaaS is particularly suitable for companies with:
- Systems with high criticality and security relevance
- Applications with frequent updates or release cycles
- Environments with strict regulatory requirements (e.g., FDA, MDR, GxP)
- Need to relieve internal resources while ensuring compliance
Conclusion and future perspective
The requirements for validation are constantly increasing due to growing system complexity, networking, data volumes, and the use of AI. At the same time, cybersecurity risks are increasing, making it essential to continuously update security mechanisms and review them against new threat scenarios.
Validation as a Service offers companies the opportunity to overcome these challenges efficiently, sustainably, and in a future-proof manner. Through continuous, compliant validation, you gain both security and freedom to innovate – while experienced experts reliably validate your systems and adapt them to new requirements, including the increasingly important areas of AI and cybersecurity.
Frequently asked questions (FAQ's)
Unlike one-time, project-based CSV, VaaS handles the continuous validation of all relevant systems. Changes, updates, and new regulatory requirements are continuously evaluated and documented to ensure that the valid state is maintained at all times.
VaaS is ideal for organisations in regulated industries such as pharmaceuticals, medical technology, cosmetics, and dietary supplements. Companies with frequent software updates, complex system landscapes, or limited internal quality management resources benefit the most.
Validation as a Service takes into account all relevant regulations and standards, including GxP, ISO 9001, ISO 13485, and FDA 21 CFR Part 11. The goal is complete compliance and auditability throughout the entire life cycle of a system.
- The service provider accompanies every release and evaluates its impact on validated systems. This means that software updates can be implemented without delay, while compliance is guaranteed at all times.
- Relief for internal resources
- Faster update cycles without compliance risks
- Consistent, documented processes across all systems
- Predictable costs thanks to fixed service contracts
- Access to specialised experts with regulatory know-how
Yes. Modern VaaS models take into account AI applications, cloud environments, and cybersecurity aspects. They ensure that even dynamic, data-driven systems comply with regulatory requirements.
VaaS providers operate according to strict security guidelines. This includes controlled access rights, encrypted communication, and complete traceability of all changes to ensure data integrity and security.