Change management (Change Control) for more data integrity and traceability
Digital Life Sciences GmbH has already been successful as a partner of d.velop AG since 2007. The managing directors and long-time employees are among the co-inventors of d.3: The d.velop documents (formerly d.3ecm) basic product is now used by over 12,500 customers as an ECM/DMS/archiving system.
The solutions of Digital Life Sciences GmbH are to be understood as a complementary additions to the basic product d.3. We offer you validatable solutions in the sectors Document Control, Training Management, Deviation Control, CAPA, Change Control or Change Management, Complaint, Contract Management and Digital Personnel File. Learn more about our professional change management and our other software solutions.
Our software solutions can be extended like modular building blocks to a complete GxP-compliant eQMS suite. They primarily address document-based manufacturing/production and quality management processes. In addition to our customers of the classical Life Sciences industry (pharmaceutical, medical technology, food supplements, cosmetics), also customers from the steel industry and the service sector trust our solutions.
Learn more about our Change Control software solution.
What are the focal points of our software solutions
With our dls | eQMS solution suite, you can archive any documents such as invoices, manufacturing records, delivery bills, quotations and much more – everything is stored in the digital central archive in compliance with regulatory requirements (GxP or GoBD). Furthermore, we have specifically expanded our archive system with regard to electronic signature solutions for GxP requirements. Thus, you can e.g. release your batch documentation and keep your batch record electronically. Keep a digital overview of change requests and the like and save on unnecessary paper.
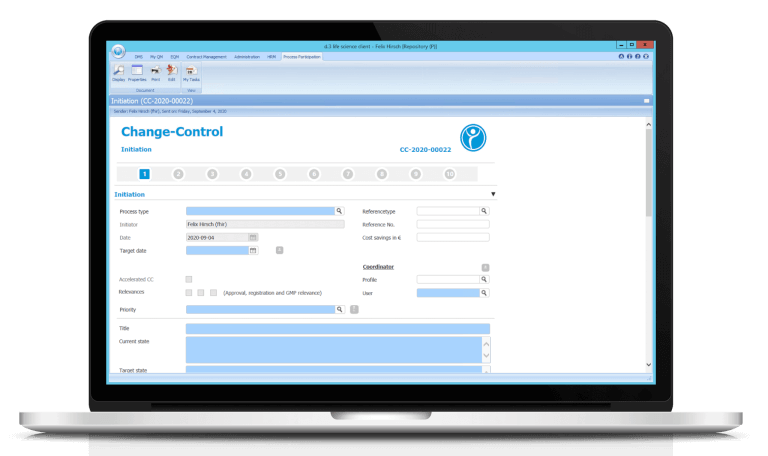
Change Control - our Change Management in detail
With our change management (Change Control) you control your QM process with our electronic workflows. The change is classified according to the meta data entered in the form. In addition, an automatically generated PDF based on the form is stored in the system after each step. Of course, we have also added our electronic signature to our QM processes.
What benefits do you get from Change Control (CC)?
By using the Change Control (CC) by Digital Life Sciences, you can significantly reduce your costs and processing times and increase the quality of your results.
Furthermore, you can make changes to products, processes or systems against the background of regulatory and internal specifications.
Change control can be linked to upstream processes, such as deviation reports or corrective and preventive actions. This facilitates the interaction of the individual management steps and helps the change process.
In terms of process reliability, the workflow system ensures that after each process step, the task is assigned to the next person in charge. An escalation system takes effect in case of missed deadlines.
Finally, every employee is entitled to view the current status of all Change Control processes, even if there is no process participation. In terms of controlling, it is ultimately possible to create reports and statistics of the CCs.
The team of Digital Life Sciences GmbH - professional, competent and helpful
If you have any further questions or require information about our other software solutions or the Change Control, the team at Digital Life Sciences GmbH will be happy to help you. Please feel free to contact us at any time. We will provide you with comprehensive consultancy.
Are you looking for a solution for your change processes?
Then take a look at our solution now