Corrective and Preventive Action (CAPA) & Batch Documentation by Digital Life Sciences GmbH
When it comes to GxP-compliant documentation solutions, Digital Life Sciences GmbH is your experienced contact. Whether pharmaceutical industry, medical technology, diagnostics, healthcare sector, food industry, steel industry or contract manufacturing – from batch documentation and Corrective and Preventive Action (CAPA) to document control, training management, deviation management (DC) through to contract management and change management (CC), we offer you individual solutions. Learn more about our professional software solution for CAPA.
We develop and market innovative software for the end-to-end digitalization of your business processes and industry-specific specialised procedures. We focus on the control of records and archiving, the control of documents as well as production-related QM processes.
Numerous satisfied customers already trust in our comprehensive solutions and our excellent service. Feel free to request a detailed consultation and comprehensive information about our digitalisation solutions.
You can reach us by telephone under 02542 – 20201 0 as well as via our contact form. We look forward to your inquiry and will be glad to help you in a competent and friendly manner.
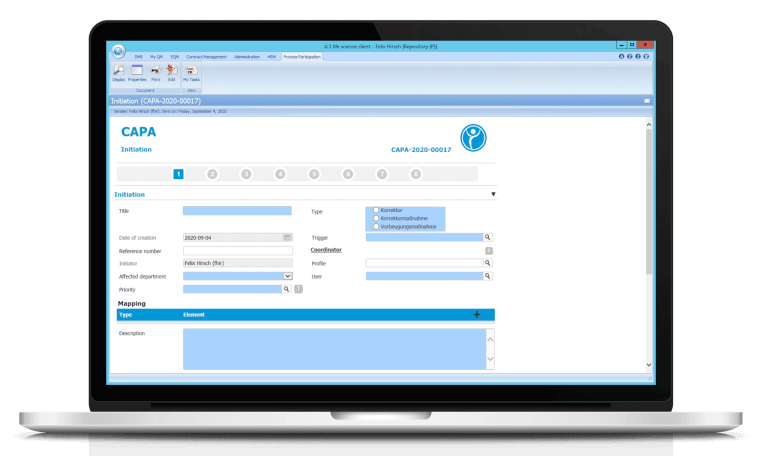
Digital Corrective and Preventive Actions (CAPA)
Corrective And Preventive Actions (CAPA) provides high data integrity. CAPAs are no longer lost because they are stored electronically from the beginning. Moreover, a high level of traceability is provided – since CAPAs can be linked to other modules such as Change Control, an easily traceable chain of interrelated individual processes is created. CAPAs can be initiated, for example, from deviation reports or the can initiate a change process.
A high level of process reliability is ensured by the fact that the CAPA is forwarded to the respective person responsible for the respective subject after each step – if time limits are exceeded, the escalation system steps in. Transparency is created by allowing every authorised employee to see the current status of all CAPA processes – even if he or she is not involved in the process. Moreover, reports and statistics can be generated about your CAPAs.
Our software enables you to create effective CAPAs that you can easily monitor and control digitally.
Regulatory requirements are for example:
- ISO 9001:2015, Chapter 8
- ISO 13485:2016, Chapter 8
- EU-GMP Guideline Part 1, Chapter 8
Digital Batch Documentation
CAPA is closely linked to digital Batch Documentation. You no longer need storage space for paper. The signature is applied electronically – therefore, a manual transport of the batch file is no longer necessary. Furthermore, the QP can release the batch documentation from their mobile device. The automatically generated batch file contains all information at a glance. Moreover, our digital Batch Documentation allows all authorised employees to view all batch documents transparently – even when they are on the road.
The digital batch documentation supports you in batch-oriented scanning. It is also possible to automatically transfer metadata to the scanned document. Adding to this, our solution enables unalterable electronic storage in accordance with the retention periods specified by the regulatory authorities and optional maintenance of your batch record.
Regulatory requirements are for example:
- EU GMP guideline part 1, section 4
Individual digitalisation solutions for every need
From corrective and preventive actions (CAPA) to batch documentation, document control, training management and deviation management (DC) through to contract management and change management (CC), we are your experienced partner for individual digitalisation solutions. Our products have been successfully audited in TÜV audits according to the guidelines of the ISO standards 9001 and 13485 as well as during inspections of the regional council (Germany) and can be individually adapted to the lifecycle of your specification documents and forms.
Over 12,500 customers worldwide are already successfully using our basic product d.3 as a central ECM/DMS/archiving system. If you archive your documents with dls | eQMS, quotations, invoices and delivery bills as well as manufacturing or test reports can be stored in the central digital archive in compliance with regulatory requirements such as GoBD or GxP.
Contact us for a detailed consultation. We would be glad to take some time for your questions and concerns and offer you many years of experience and know-how.
Are you looking for a solution for your CAPA processes?
Then take a look at our solution now