From individual solutions to a global eQMS: How SCHOTT is digitising processes with dls | eQMS
Whether pharmaceutical vials for corona vaccines, Ceran cooktops or mirrors for the world’s largest telescope – SCHOTT AG’s high-tech products are used in various areas of life around the world. Headquartered in Mainz, Germany, the company has been one of the innovation leaders for specialty glass and high-tech materials for over 130 years.
But international success is not based solely on state-of-the-art production technology. Efficient, transparent and secure processes in the background are just as important. SCHOTT’s success story impressively demonstrates how a traditional company with a clear vision and a strong partner was able to reorganise its quality management.
This blog post explains how SCHOTT managed to migrate more than 50,000 documents from various systems to the electronic document and quality management system dls | eQMS from Digital Life Sciences within just a few months – and thus create the basis for a globally standardised, future-proof quality management system.
From glass innovation to global corporation
Innovation needs secure processes
Initial situation: Inconsistent systems, high complexity
Prior to the introduction of dls | eQMS, various business units, corporate functions and accredited test laboratories at different locations around the world used different systems for document management, including:
- Advanced Optics
- Hometech
- Lighting and Imaging
- Research & Development (R&D)
Each unit selected its software independently, set up its own structures and defined individual processes. There was no central control system.
In order to increase efficiency and ensure quality worldwide, binding specifications were required. The aim was to provide the specialist departments with a standardised document control system while at the same time meeting the specific requirements of the production environments and corporate functions.
A further deficit was identified in the area of employee training. Previously, this was done by e-mail, Excel list or paper folder. An approach that was neither efficient nor sustainable.
The consequences:
- Inconsistent document structures between departments
- Manual training administration (Excel lists, e-mails, paper folders)
- High administrative effort to comply with regulatory requirements
- Lack of transparency regarding document status, employee qualifications and training status
“Ultimately, all units struggled with the same document control challenges. We wanted to centralise and harmonise this,” summarises Christine Strubel, Head of Management Systems Quality & EHS.
However, in order to solve this challenge in the long term, a solution was needed that would not only reduce the previous complexity, but also lay the foundation for a standardised, transparent and future-proof quality management system at SCHOTT. Two key aspects had to be taken into account: A system had to be found that took into account the requirements of the individual units and corporate functions as well as SCHOTT’s IT policy.
In close cooperation with SCHOTT’s IT department, the decision was made in favor of a solution that combines both aspects. A system that connects all units, reliably fulfills regulatory requirements and makes the daily work of employees noticeably easier.
The solution: Introducing the dls | eQMS
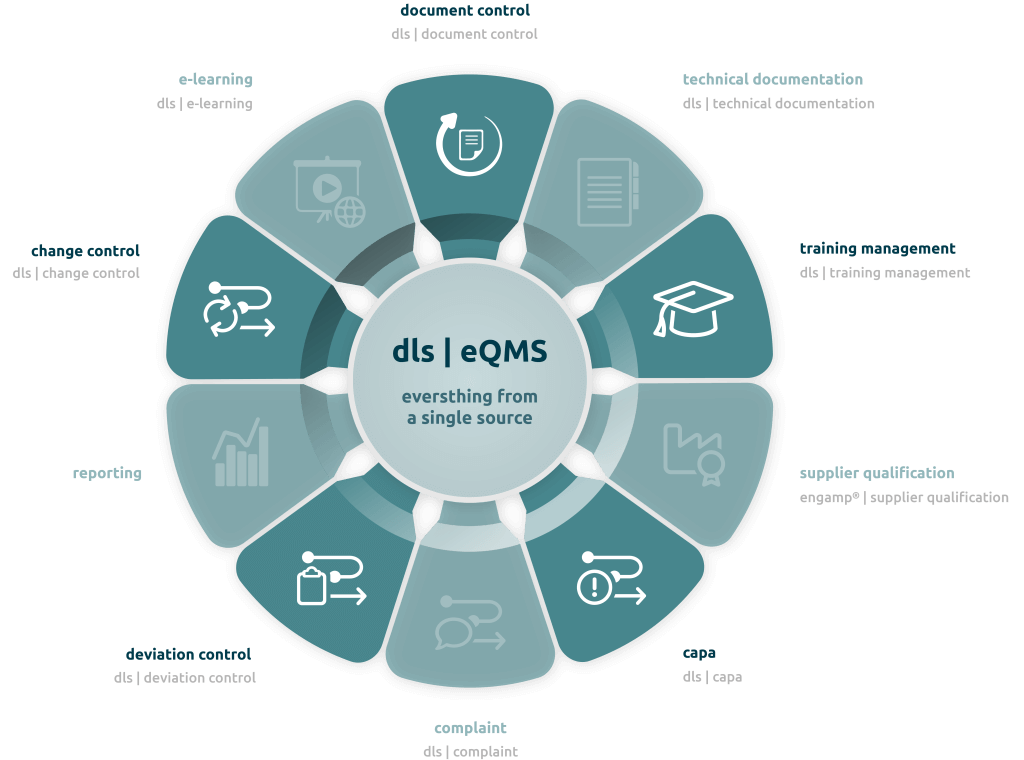
In January 2018, SCHOTT contacted Digital Life Sciences GmbH, an expert in GxP-compliant documentation solutions. The focus was on the electronic document and quality management system – “dls | eQMS” (formerly d.3 eQMS), which was intended to fulfill precisely these requirements and more:
- Central document control: role- and workflow-based search and access options for digital information, independent of departments and business units
- Training management: digital training administration with electronic exams
- Global usability: multilingualism and worldwide availability for different locations
- High usability: modern user interface that ensures user acceptance
- Compliance: fully validated system in accordance with requirements, e.g. GAMP®5, EU Directive
- Quality management: trouble-free fulfillment of documentation and quality management obligations
- Modularity: additional functionalities through the use of further modules, such as
- Change Control (change management)
- CAPA Management – Corrective And Preventive Action, a practical part in quality management and part of GMP-compliant work (“Good Manufacturing Practice”)
- Deviation management (Deviation Control)
The system should also offer good value for money without the need for expensive individual programming.
System overview of the dls | eQMS modules
The start of the project: Consolidation and pilot phase
In September 2018, two business units began introducing the dls | eQMS system. In close cooperation with Digital Life Sciences, SCHOTT worked intensively on merging the documents.
The biggest challenges here:
- Migration of over 50,000 documents from six legacy systems
- Definition of clear role and authorisation concepts
- Introduction of new workflows that are both regulatory compliant and practical
- Validation of the system to meet regulatory requirements
The first two units, the accredited test laboratories and the Lighting and Imaging business unit, successfully went live in January 2020, after a project duration of just one and a half years.
“The experience we had was extremely good. I would also like to highlight the validation of computer-aided systems, in which Digital Life Sciences has helped us significantly,” emphasises Strubel.
Dr. Dennis Sandkühler, Director Quality at DLS, also emphasises: “We have always oriented ourselves to our own standards when setting up. We didn’t want to bend ourselves or the business units, and we certainly didn’t want to change the entire organisation.
This illustrates the importance of a trusting partnership and a sure instinct when migrating existing data to a modern document management system.
Project progress & milestones: From pilot project to global rollout
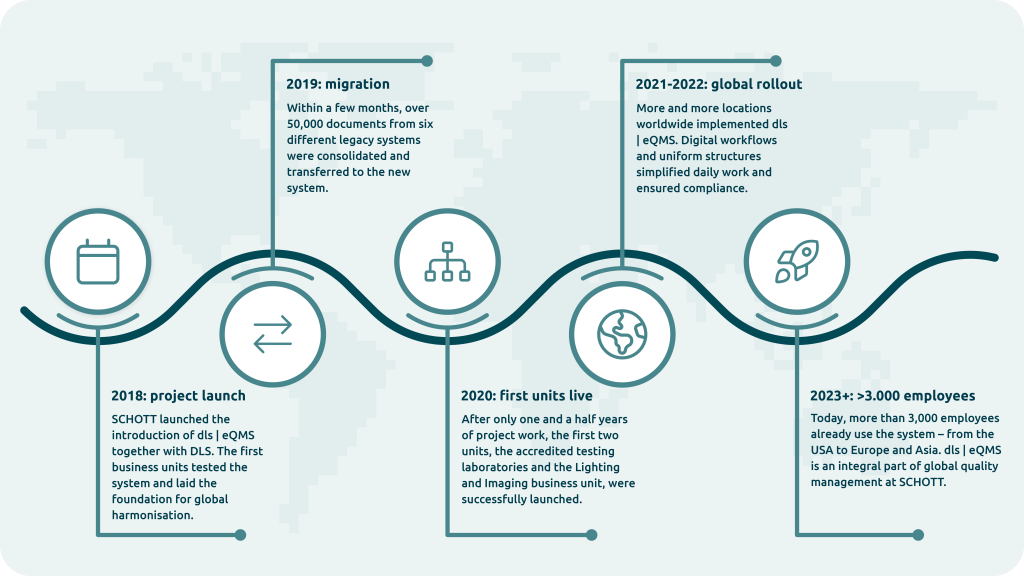
The successful pilot phase was followed by the expansion to other business units. Today, more than 3,000 employees worldwide already work with dls | eQMS – from the USA to Europe and Malaysia. Christine Strubel says: “We have so many new possibilities with the dls | eQMS that simply didn’t exist before.”
The advantages are clearly noticeable:
Centralised document control: A uniform structure, transparent processes and a reliable full-text search ensure that SCHOTT employees always work with the latest version of a document. This increases the quality and speed considerably and makes the time-consuming sending of e-mails superfluous.
Digital workflows through role and rights management: The optimised approval and checking processes now run smoothly. In the event of an absence, responsibility is automatically transferred to the next person in charge to ensure a smooth workflow.
Structured deviation and change management: dls | eQMS logs, corrects and tracks deviations in order to meet the requirements of DIN EN ISO/IEC 17025 and GMP requirements.
Training Management: SCHOTT now has a centralised, digital training management system. Employees are trained in real time with relevant documents. Digital electronic exams, proofs and tests ensure transparency and efficiency.
“Training management in particular helps us enormously,” says Strubel, “We didn’t have that before, we kept track of it manually in an Excel file. […], now we have a very nice overview in the dls | eQMS system. It’s also very well received by the employee.”
Conclusion: A step into the digital future
After almost five years of using dls | eQMS, SCHOTT’s assessment is consistently positive.
The company has harmonised processes worldwide, created transparency and laid the foundation for further growth through the use of central document control, training management and deviation and CAPA management.

“We have chosen the right product. Our high demands on usability have been met, the search function in dls | eQMS alone is excellent. That wasn’t bad in our previous system either, but it’s better now. After the trainings and the familiarisation period, it is now much easier to manage all documents,” Strubel concludes.
In the pharmaceutical and medical industries, the efficiency and transparency of a company’s processes are crucial to its success, in addition to the quality of the end product.
For SCHOTT today, as in 1879, innovation does not stop with the product – it permeates the entire company.
Frequently asked questions (FAQ's)
- Who is SCHOTT AG?
SCHOTT AG is an international technology company based in Mainz, Germany, with more than 130 years of experience in specialty glass and high-tech materials. The company has 34 branches and around 16,500 employees worldwide and generated sales of over 2.5 billion euros in 2021.
Today, SCHOTT supplies products that are used both in everyday life and in high-tech industries – from ceramic hobs and mirrors to syringes, vials and ampoules. This diversity makes SCHOTT an important partner in markets with high quality and safety requirements
- Why is document management so important for SCHOTT?
In highly regulated industries such as pharmaceuticals or medical technology, high product quality alone is not enough. Processes must be documented in a transparent, comprehensible and legally compliant manner. Efficient document management is crucial for complying with regulatory requirements and ensuring that employees always work with up-to-date information.
- What problems did SCHOTT have before the introduction of the electronic quality management system (eQMS)?
Prior to the introduction of the new system, the various company divisions each used their own document management solutions. This led to inconsistent structures and a high administrative burden. Employee training was documented manually using Excel lists, emails or even paper folders. This approach was not only inefficient, but also error-prone. In addition, there was no central overview of the employees’ qualification level and training status, which posed a considerable risk to compliance with quality standards.
- Why was a centralised solution necessary?
A centralised solution was needed to create uniform global standards and increase efficiency. A standardised system ensures that documents are always available in the latest version, that regulatory requirements are reliably met and that training is centrally managed and transparently documented. This is the only way to establish a harmonised and future-proof quality culture throughout the company.
- Why was the dls | eQMS chosen?
The system fulfills all requirements: central document control, worldwide usability, multilingual interface, high user-friendliness and complete validation in accordance with the applicable regulations, such as GAMP®5 and the EU directives. It is modularly expandable and can be adapted to the specific needs of SCHOTT.
- What features does dls | eQMS offer?
The dls | eQMS has numerous features that make everyday work easier. This includes central document control with automatic versioning, digital workflows for release and training management for the digital mapping of trainings and proofs. Deviations and changes can be systematically tracked, while CAPA management supports preventive and corrective measures. A powerful full-text search enables quick access to relevant documents.
- What role did the collaboration with Digital Life Sciences play?
The close collaboration with Digital Life Sciences was crucial: DLS supported SCHOTT with data migration, workflow modeling and system validation. This enabled the implementation of a user-friendly, globally applicable and regulatory reliable eQMS.
- How did the introduction of dls | eQMS go?
The project started in 2018 with a pilot phase in two selected business units. After successfully consolidating the documents and defining new workflows, the first units went live at the beginning of 2020. The system was then gradually rolled out in other areas, ensuring that it is now used globally – from the USA to Europe and Malaysia. The system was set up so that English is a mandatory language to facilitate international communication. The user interface can also be displayed in German.
- What were the challenges of migration?
The migration presented SCHOTT with a major challenge: over 50,000 documents had to be transferred from different systems and duplicates had to be avoided. At the same time, it was required to define clear role and permission groups in order to be able to securely control access to sensitive documents. A key step was the implementation of new workflows, which had to be both regulatory compliant and practical. The validation of the system required close coordination between the project teams and the experts from Digital Life Sciences.
- How many employees use the system today?
More than 3,000 employees worldwide now work with dls | eQMS and benefit from standardised structures, more efficient workflows and improved transparency. The introduction of the system has thus long since developed into a global success model that has left the pilot phase far behind.
- What advantages does SCHOTT have with the eQMS?
The introduction of the eQMS brings SCHOTT numerous advantages. It ensures uniform and transparent document management so that all employees always work with the latest version. Manual Excel lists and e-mail reconciliations are no longer necessary, making processes more efficient and less error-prone. Audit-compliant processes increase compliance, speed up releases and ensure standardised quality. The eQMS harmonises quality processes worldwide and creates the basis for future-proof quality management.
- How does training management improve work?
Training Management has completely digitalised the previously manual organisation of trainings. Employees are automatically notified as soon as new or updated documents are available. They can complete trainings directly in the system and prove their participation via digital exams and tests. This provides an up-to-date overview of the qualification level, which saves time, reduces errors and fulfills regulatory requirements.
- What is SCHOTT’s conclusion after five years of use?
After almost five years of usage, SCHOTT’s assessment is consistently positive. The eQMS has standardised processes worldwide, ensured greater transparency and sustainably increased efficiency. Particularly noteworthy are the high level of user-friendliness and the powerful search function, which make everyday work much easier. This means that SCHOTT not only fulfills its own high standards, but has also created the basis for further growth and a future-proof quality culture.