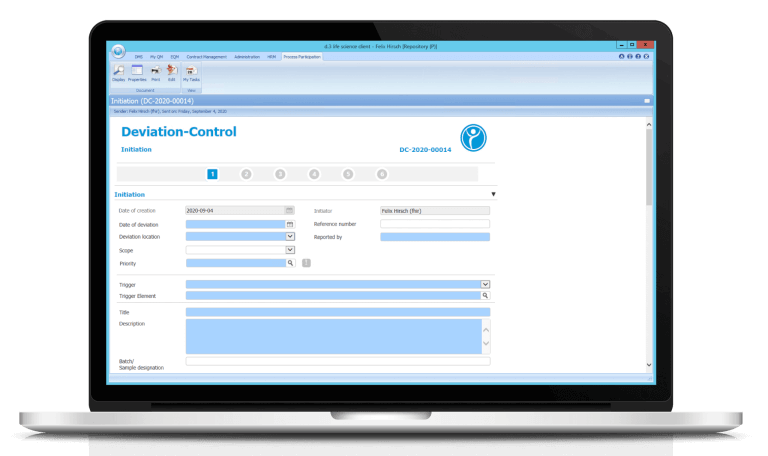
Digitalize your ISO processes with electronic deviation management / Deviation Control
If you intend to digitalize your paper-bound ISO processes, Digital Life Sciences GmbH will be glad to assist you with its experience and software solutions. Whether Deviation Control (DC) Change Management (CC) or Corrective and Preventive Actions (CAPA) – with our software solutions, you can digitally implement your processes from capture to completion. Learn more about our professional deviation control software.
We are your partner for GxP-compliant documentation solutions – Digital Life Sciences GmbH develops and markets software for the seamless digitalization of business processes and industry-specific specialist procedures. Our focus is on the control and archiving of records, the control of documents and production-related QM processes.
Discover our service portfolio and our competent customer service for yourself. If you have any questions, we’d be pleased to give a detailed consultation.
Deviation Control ensures transparency & process reliability
Conduct your previously paper-based process electronically and take advantage of the numerous benefits in Deviation Control with our deviation management software.
Deviation reports are stored electronically from the outset and can be linked to other modules such as Corrective and Preventive Actions (CAPA) and Change Management (CC). This makes it easy to trace interrelated individual processes. Furthermore, our solutions for Deviation Control allow significantly lower process runtimes.
After each step, the workflow system ensures that deviation reports are forwarded to the respective person in charge. The escalation system takes effect in case of missed deadlines. This simplifies your quality assurance.
Deviation Control also means that every authorized employee can see the current status of all deviation reports – even if he or she is not involved in the process. This ensures a high level of transparency. Moreover, reports and statistics can be generated on your deviation reports, e.g. as support for your PQR.
Deviation Control offers numerous advantages
Our digital deviation management solutions enable automatic PDF creation and storage of the form in the eDMS after each step and automatic classification of the deviation based on the metadata entered in the form. Moreover, the master data can be referenced from the ERP system and documents can be added.
Individual tasks can be assigned for further processing and escalation messages can be sent both within the system and by e-mail.
Regulatory requirements are for example:
- ISO 9001:2015, Chapter 8
- ISO 13485:2016, Chapter 8
- EU-GMP guideline part 1, chapter 8
QM processes accompanying production
With our electronic workflows, you can easily control processes such as deviation management (Deviation Control), Corrective And Preventive Actions (CAPA) and change management (Change Control). Benefit from effective support in creating reports and generate statistics on the frequency, criticality or runtime of your QM processes. In order to increase transparency and ability to provide information in your company, authorized employees also have access to the running processes that are not involved in the process.
We offer you solutions developed against the regulatory background
Whether document control, training management, Deviation management (Deviation Control), Change Management (CC) or Corrective and Preventive Action (CAPA) or contract management – we offer digitalization solutions specially developed against the regulatory background. Simply tell us your requirements and needs – we will be happy to adapt our solutions to the lifecycle of your specification documents and forms.
Thousands of customers successfully use our basic product d.3 as their central ECM/DMS/archive system, many of them in the pharmaceutical industry. The solutions we offer are complementary additions to the basic d.3 system and are aimed at document-based manufacturing/production and quality management processes.
If you have any questions about our range of services, we will gladly answer them and advise you in detail about your options. Just contact us – we look forward to your inquiry and will help you in a competent and friendly manner.
Are you looking for a solution for your deviation processes?
Then take a look at our solution now