From paper to platform – QMS in the digital transformation of the pharmaceutical industry
Many companies in the life sciences sector still rely on paper-based QM processes today. However, these analogue systems are reaching their limits in the face of growing requirements and increasing complexity. In accordance with our regulatory requirements, complete documentation and traceability are required.
In a digital world where speed, efficiency and compliance are equally important, a digital quality management system plays a key role. Nowadays, quality is not just a matter of control, but a strategic step for the long-term success of a company.
In this article, you can find out what role a digital quality and document management system (eQMS/eDMS) plays in digital transformation and how companies can benefit from it in the long term.
Status quo of the industry
As part of Bitkom Research 2023, companies from various sectors were asked how important digitalisation is to them and how satisfied they are with digital progress:
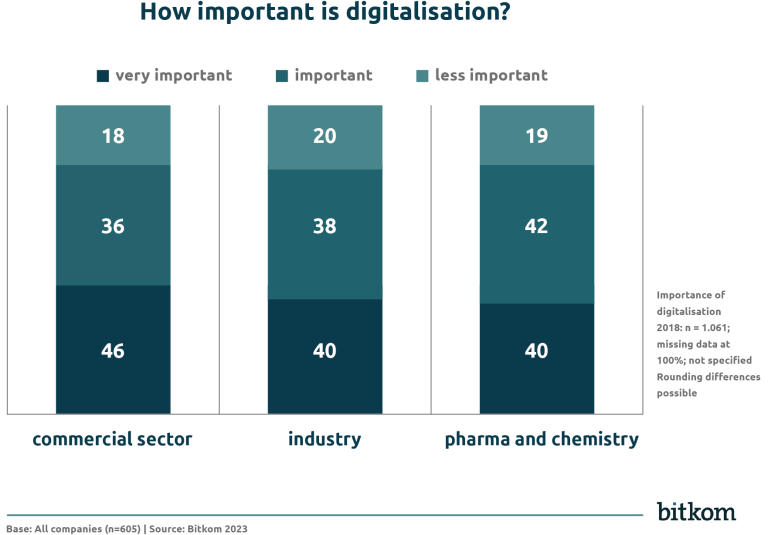
- In the industrial sector, 78% of companies consider digitalisation to be important or very important.
- In the commercial sector, it is 76%.
- In the pharmaceutical and chemical industries, 82% of companies state that digitalisation is important to very important.
Despite this high percentage, the pharmaceutical and chemical industries are less likely to make digitalisation a top priority. Industry and commerce are prioritising the issue much more strongly.
These are the results regarding satisfaction with digitalisation:
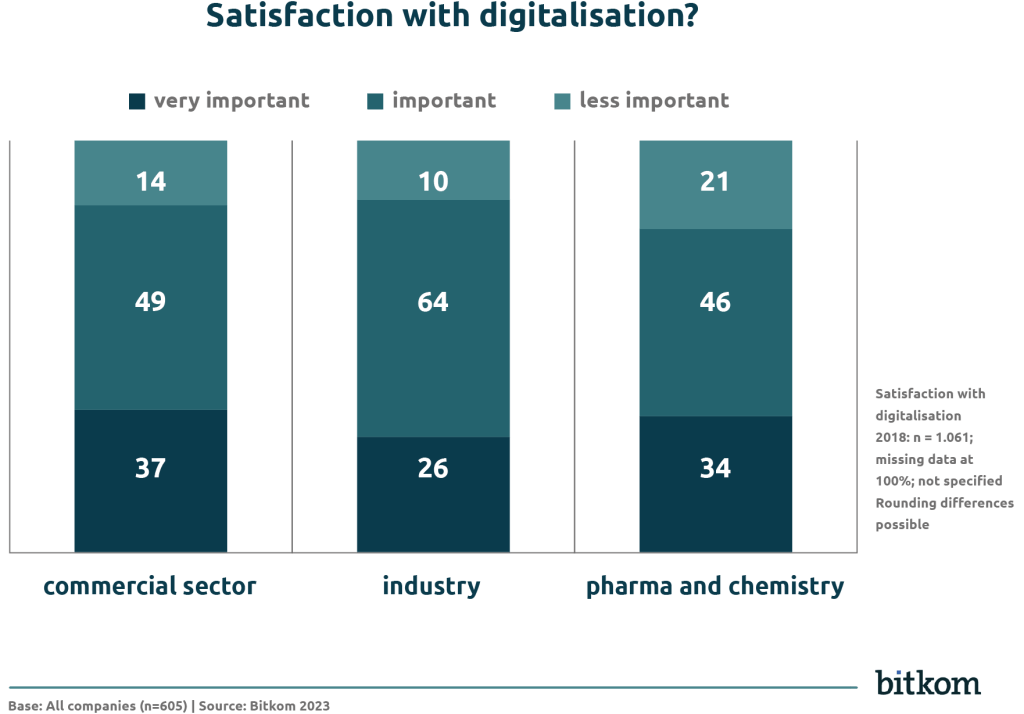
- 90% of industrial companies are satisfied or very satisfied.
- The commercial sector follows with 86%.
- In the pharmaceutical and chemical industries, satisfaction is significantly lower at 80%. Around 21% of companies in this sector express a high level of dissatisfaction with their digital development.
This high level of dissatisfaction shows that there are challenges when implementing new systems, e.g. due to regulatory requirements or acceptance within the organisation.
With a digitalisation index of 50 points, the pharmaceutical and chemical industry is just below the average for industry as a whole. The industry predicts that the level of digitisation will continue to fall until 2023.
DMS & QMS in the digital transformation
For many companies, digitisation still only means scanning paper documents and filing them in folder structures. But digitalisation goes far beyond that. For us, it means the targeted integration of digital technologies into products, processes and services.
Documents form the basis of almost all quality-relevant processes in the regulated environment – in line with the principle: “What wasn’t documented, wasn’t done.” Only comprehensible documentation can be used as proof.
In this context, electronic systems such as eDMS and eQMS play a central role:
The document management system (eDMS) is the basis for digital transformation. It enables companies to record, store, manage and distribute their documents systematically, digitally and in compliance with the law. This includes, for example, verification documents, test protocols and release protocols, certificates of analysis, reports, forms and much more.
Building on this, the electronic quality management system (eQMS) acts as a central platform. It supports the creation, review, approval, release, training and legally compliant implementation of documented specifications such as SOPs, work and procedural instructions.
Structure of modern eDMS & eQMS – modular, scalable, intuitive
The introduction of an electronic eDMS & eQMS is based on a modular building block model. The structure is scalable, needs-based and precisely tailored to the requirements of each customer.
The focus is on an integrated platform in which the eDMS with standardised data models forms the digital foundation. Qualified, validated and standardised modules can be flexibly set up on top of this, such as for Document Control, Training Management or QM Workflows as well as many other solutions. All modules can be individually configured.
The eQMS can be introduced on a modular basis and expanded like a building block model to create a fully comprehensive, GxP-compliant eQMS suite. Thanks to the integration of the ECM/DMS system, both GxP and non-GxP applications can be mapped in almost all document-based company processes. The connection to existing ERP systems, such as SAP, enables a continuous flow of information across system boundaries.
Differentiated user management with a roles and rights concept is also important, as this allows responsibilities to be clearly mapped and regulatory requirements to be met.
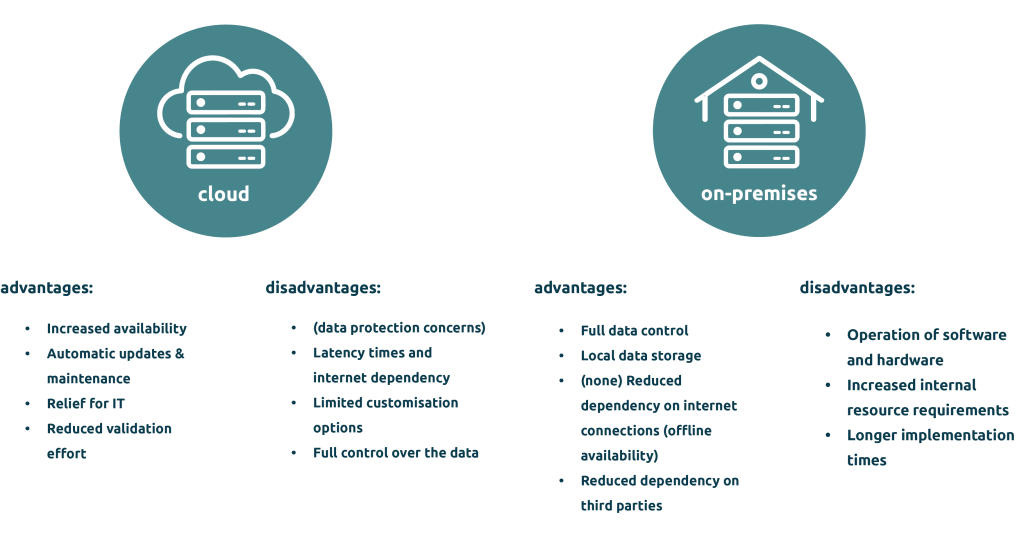
eDMS & eQMS provision forms: On-premises or cloud?
Risks under control, challenges in mind – digitalisation should not be underestimated
The change from paper-based to digital processes offers companies numerous opportunities, but is also associated with risks and challenges. Various technical, organisational and personnel requirements must be met in order to make the changeover a success.
Data protection and data security: Systems must be protected against unauthorised access and data must be encrypted according to access controls. Companies can choose between on-premise and cloud solutions to ensure that their documents are stored securely at all times.
Validation: In a regulated environment, the validation of computerised systems is essential. All changes, tests and approvals require complete documentation. Any customisation that may affect the integrity of the overall system must be revalidated during system updates.
Compliance and regulatory requirements: A digital QMS must consistently implement industry-specific regulations. This includes, for example, document control, audit trails and the use of digital signatures to ensure audit compliance and legally compliant processes.
Change management: The biggest challenge is the change itself. The switch to digital systems is often met with resistance – especially if employees have been used to paper-based processes for years. Early involvement, open communication and training help to increase acceptance and reduce uncertainty.
Employees and staff: Due to the shortage of skilled workers, many companies are faced with limited capacity for the introduction of new systems. In the run-up to this, personnel scope must be created in good time and clear roles defined, for example: Who is a key user, administrator or responsible for training?
Qualification and training of staff: Right from the start, it is important to provide employees with targeted training so that they can make optimum use of the system. Thanks to the direct integration of the training program into the eQMS, learning content can be seamlessly integrated.
Resources: An appropriate budget is required for the introduction and maintenance of digital systems so that they can be successfully operated and further developed in the long term.
More than just digital – advantages of an eDMS & eQMS
An electronic quality and document management system offers numerous advantages, especially in regulated sectors such as the pharmaceutical industry:
Improved compliance: Automatic versioning, digital signatures and predefined approval processes ensure compliance with regulatory requirements. This enables pharmaceutical companies to fully proof their compliance.
Increased data integrity and traceability: All changes to documents are recorded transparently in the audit trail. Integrated traceability management protects against unauthorised interventions and enables complete traceability.
Reduction and redundancies: Duplicate records and incorrect versions are a thing of the past. A central template directory provides standardised document templates that can be stored and managed there. Automated workflows and approval processes prevent duplicate processing and ensure clear, traceable workflows.
Time and cost savings: Significantly reduced paper consumption, lower archiving costs and faster processes lead to tangible savings.
Audit readiness: Regulatory-related documents can be made available in audits at any time at the touch of a button.
Increased efficiency through automation: Automatic escalations and notifications of deadlines ensure smooth processes, minimise manual intervention and shorten throughput times.
Centralised data management: All quality-relevant documents are stored centrally and can be seamlessly linked to systems such as ERP, PLM or LIMS.
Improving cooperation and communication: Employees can work on documents and approval processes simultaneously and access content across locations.
Faster decision making: The direct insight into current and valid documents eliminates the time-consuming search for information. This speeds up the decision-making process and increases transparency.
Scalability and future-proofing: The system can be introduced on a modular basis and expanded individually step by step.
Practical example: ChemCon – from paper chaos to efficiency
ChemCon GmbH is a medium-sized company with around 130 employees that specialises in the production of active pharmaceutical ingredients and fine chemicals.
Initial situation and challenges
Before the digital system was implemented, quality management was completely paper-based, unstructured and error-prone. This process was characterised by a high level of manual effort, time-consuming document updates and complex training management.
The solution
To optimise the processes, the dls | eQMS was introduced with the modules Document Control and Training Management. The solution enabled the digitalisation of central quality processes and created the basis for greater efficiency, transparency and traceability.
The results
- Up to 40% time savings in documentation
- Faster and more precise updating of documents
- Transparent and optimised training management with clear proof
By switching to the dls | eQMS, ChemCon was able to significantly increase its efficiency and at the same time reliably meet regulatory requirements.
Digital quality management as an innovative force for the future
The future of quality management is digital, intelligent and networked. New trends and technologies such as AI-supported quality processes, mobile solutions for access from anywhere and smart workflows with ChatGPT integration open up completely new possibilities in terms of efficiency, data usage and collaboration.
However, a digital platform, such as a GxP-compliant eQMS/eDMS system, is a prerequisite for these innovations to be used in the future. Many companies are still paper-based and only have a digital archive solution. An enormous potential that often remains untapped.
A well-thought-out digitalisation strategy and the introduction of an integrated eDMS/eQMS system are essential to accompany companies safely on the path to digital transformation. Companies that invest in digital quality processes at an early stage and actively involve their employees strengthen their global competitiveness and ensure their long-term business success.
The path to a digital future begins with the first step towards future-proof quality management, which does far more than just documentation. When used strategically, the eQMS becomes the key to sustainable innovation and a real competitive advantage.
For more information on our solutions, please visit: https://www.digital-ls.de/en/solutions/