Table of Contents
BM-Flow reaches end-of-life – what now?
Why future-proof eQMS systems are crucial and how to act now
biomedion GmbH has finally discontinued active support for the electronic quality management system (eQMS) BM-Flow as of December 31, 2024. BM-Flow has been in sunset mode since the beginning of 2023 and is now being fully discontinued. For companies that continue to use BM-Flow productively, this is a critical point – both from an IT and compliance perspective.
After all, eQMS systems are important tools that form the foundation of numerous digitalised quality processes in regulated industries such as pharmaceuticals, biotechnology and medical technology. A functional QMS is important as it ensures integrity, auditability and regulatory compliance.
A change of provider in the eQMS sector is rare and should always be carried out professionally and with foresight. Companies that take action now can significantly reduce risks such as security gaps and audit deviations. At the same time, this upheaval opens up the opportunity to modernise processes, close security gaps and create a solid basis for future growth.
In this blog post, we explain why the discontinuation of support for BM-Flow is creating pressure to act and how companies can use this time strategically to future-proof their quality management.
Why eQMS systems are rarely changed – and what that says about their significance
The introduction of an electronic quality management system usually takes place with a long-term perspective that often lasts for many years. This is due to the fact that eQMS systems are deeply rooted in the organisation: They integrate processes, SOPs and user roles and therefore have a significant impact on day-to-day work and corporate culture.
There are good reasons for this:
- System validation in accordance with GAMP®5 is time-consuming and ties up internal and external resources.
- Processes, SOPs and user roles are integrated into the system.
- Employees are trained and their daily work is aligned with the digital workflows.
A change therefore does not just entail a technical migration, but also a far-reaching intervention in the day-to-day practice of quality assurance. This can potentially have an impact on product quality, audit security and regulatory compliance.
The effort required for revalidation, training and integration into existing systems is considerable. A change is therefore usually only considered if it is unavoidable – as in the case of BM-Flow. This makes it all the more important that eQMS systems are not only sustainable today, but also in the long term.
Now is the right time for a sustainable solution
In the current situation, the question is not only how existing data from BM-Flow can be migrated securely. Rather, it is important to clarify: Which system will ensure the future of my quality management?
A well-founded decision depends on many factors:
- Reliability of the provider
- Long-term product strategy
- Industry experience in regulated markets
- Modular, expandable design
- Regular further development & Adaptation to new circumstances
- Availability of validation documents & audit trails
- and much more
A modern, digital quality management system must not only meet current requirements, but also be future-proof and scalable. It should integrate seamlessly into existing IT structures, meet defined security standards, secure data reliably and require as little maintenance as possible.
A structured and low-risk migration process with clear communication and minimal effort for IT and quality management departments is also crucial.
A sustainable system change begins with choosing a reliable partner. This person should not only provide technical support for the implementation, but also have in-depth industry knowledge and validation expertise as well as a clearly comprehensible migration concept.
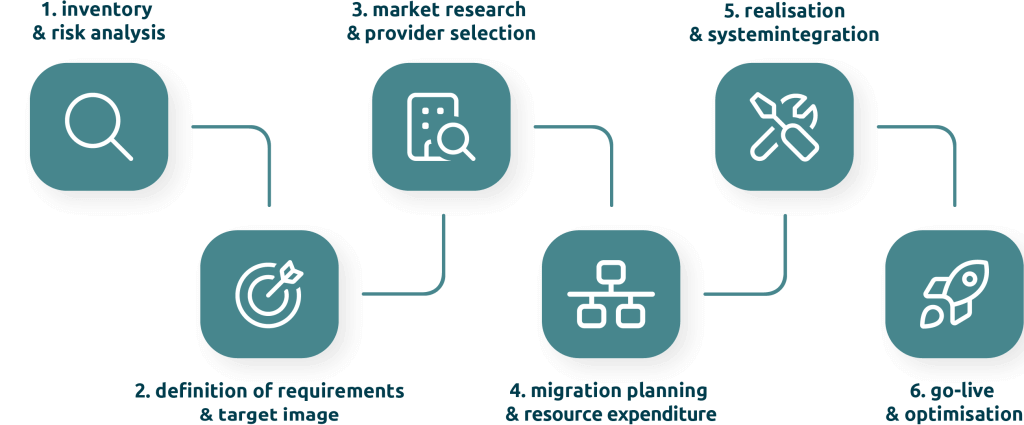
6-step roadmap for a successful eQMS system change
Migration security: Experience matters more than theory
In practice, there are clear advantages for providers with multiple migration experiences from BM-Flow: They have robust processes and well-established routines – a decisive factor in the life sciences industry with its high requirements for validation, data integrity and user rights.
Important questions are:
- What experience has been gained with mapping existing workflows?
- Are metadata, versioning and user logs reliably transferred?
- Are templates for validation documents (e.g. installation qualification (IQ), functional qualifications (OQ), performance qualification (PQ)) available?
- How are technical and specialist contacts involved in the migration process?
Experienced providers can reduce the migration effort for your organisation to a minimum – and at the same time guarantee a high level of security.
A carefully developed migration approach identifies potential pitfalls at an early stage and protects the ongoing quality process. Every project step can be tracked transparently with the help of a structured migration plan. Potential risks can be identified and minimised at an early stage.
From mandatory change to strategic opportunity
The discontinuation of an existing system may initially appear to be a burden – but it offers the opportunity for strategic realignment, particularly in the central area of quality management.
Because modern eQMS systems are far more than just document repositories with version control. They enable, among other things:
- integrated QM processes such as deviation control, CAPA (corrective and preventive actions), change control, …
- automated training workflows,
- digital supplier qualification,
- decentralised cooperation on quality-relevant content,
- seamless audit preparation and implementation,
- analytical evaluations for continuous improvement.
What matters now: Orientation, experience, vision
The selection of a new eQMS provider should be made carefully – ideally with partners who have a comprehensive understanding of the regulatory environment and have already successfully implemented comparable projects.
If experience from similar migrations is available, companies can rely on a proven process model. This reduces uncertainties, shortens project runtimes and quickly provides a validated and audit-compliant system.
Early consultation is recommended for:
- Recording the initial situation in a structured manner
- Analysing potential migration paths
- Evaluating suitable system options
- Realistic resource planning
A structured consulting process helps to identify technical and organisational risks at an early stage and to strengthen internal acceptance for the system change. Realistic planning also helps to avoid bottlenecks and save resources. This creates the necessary flexibility to be able to react confidently to unexpected challenges.
Conclusion: Set the course now for future-proof quality management
When systems such as BM-Flow are discontinued, it is more than just a technical intervention – it is a signal to rethink the digital quality strategy.
Organisations that act at an early stage make the change safe and use it as a starting point for sustainable improvements in their quality management system (QMS).
Choosing wisely, migrating in a structured way and relying on experienced partners not only ensures compliance – but also future viability.
Use the system change as an opportunity to holistically optimise your quality processes with an eQMS that grows with your requirements – so you are optimally prepared for future regulatory challenges.
Would you like to find out how a migration from BM-Flow or another system can work in your specific case?
Then we recommend that you seek a personal consultation with experienced specialists.
Source: https://www.biomedion.com/biomedion-news-events-blog/neuronos-news/closing-our-edms-system-bm-flow
Now is the right time to carry out an initial system scan, take an inventory of central QM processes and define priorities for a migration. This creates a clear roadmap instead of hectic ad hoc reactions.